Controlled Release of H2S from Biomimetic Silk Fibroin-PLGA Multilayer Electrospun Scaffolds
- PMID: 36749903
- PMCID: PMC10015463
- DOI: 10.1021/acs.biomac.2c01383
Controlled Release of H2S from Biomimetic Silk Fibroin-PLGA Multilayer Electrospun Scaffolds
Abstract
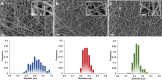
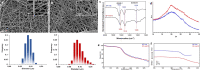
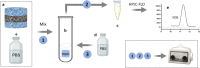
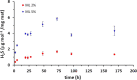
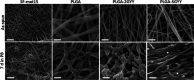
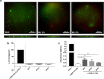
The possibility of incorporating H2S slow-release donors inside biomimetic scaffolds can pave the way to new approaches in the field of tissue regeneration and anti-inflammatory treatment. In the present work, GYY4137, an easy-to-handle commercially available Lawesson's reagent derivative, has been successfully incorporated inside biomimetic silk fibroin-based electrospun scaffolds. Due to the instability of GYY4137 in the solvent needed to prepare silk fibroin solutions (formic acid), the electrospinning of the donor together with the silk fibroin turned out to be impossible. Therefore, a multilayer structure was realized, consisting of a PLGA mat containing GYY4137 sandwiched between two silk fibroin nanofibrous layers. Before their use in the multilayer scaffold, the silk fibroin mats were treated in ethanol to induce crystalline phase formation, which conferred water-resistance and biomimetic properties. The morphological, thermal, and chemical properties of the obtained scaffolds were thoroughly characterized by SEM, TGA, DSC, FTIR, and WAXD. Multilayer devices showing two different concentrations of the H2S donor, i.e., 2 and 5% w/w with respect to the weight of PLGA, were analyzed to study their H2S release and biological properties, and the results were compared with those of the sample not containing GYY4137. The H2S release analysis was carried out according to an "ad-hoc" designed procedure based on a validated high-performance liquid chromatography method. The proposed analytical approach demonstrated the slow-release kinetics of H2S from the multilayer scaffolds and its tunability by acting on the donor's concentration inside the PLGA nanofibers. Finally, the devices were tested in biological assays using bone marrow-derived mesenchymal stromal cells showing the capacity to support cell spreading throughout the scaffold and prevent cytotoxicity effects in serum starvation conditions. The resulting devices can be exploited for applications in the tissue engineering field since they combine the advantages of controlled H2S release kinetics and the biomimetic properties of silk fibroin nanofibers.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Zhou J.; Cao C.; Ma X.; Hu L.; Chen L.; Wang C. In vitro and in vivo degradation behavior of aqueous-derived electrospun silk fibroin scaffolds. Polym. Degrad. Stab. 2010, 95, 1679–1685. 10.1016/j.polymdegradstab.2010.05.025. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

