Impaired calcium handling mechanisms in atrial trabeculae of diabetic patients
- PMID: 36750180
- PMCID: PMC9904963
- DOI: 10.14814/phy2.15599
Impaired calcium handling mechanisms in atrial trabeculae of diabetic patients
Abstract
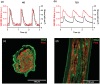
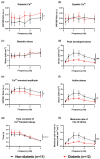
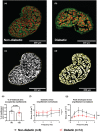
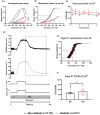
The aim of this study was to investigate cardiomyocyte Ca2+ handling and contractile function in freshly excised human atrial tissue from diabetic and non-diabetic patients undergoing routine surgery. Multicellular trabeculae (283 ± 20 μm in diameter) were dissected from the endocardial surface of freshly obtained right atrial appendage samples from consenting surgical patients. Trabeculae were mounted in a force transducer at optimal length, electrically stimulated to contract, and loaded with fura-2/AM for intracellular Ca2+ measurements. The response to stimulation frequencies encompassing the physiological range was recorded at 37°C. Myofilament Ca2+ sensitivity was assessed from phase plots and high potassium contractures of force against [Ca2+ ]i . Trabeculae from diabetic patients (n = 12) had increased diastolic (resting) [Ca2+ ]i (p = 0.03) and reduced Ca2+ transient amplitude (p = 0.04) when compared to non-diabetic patients (n = 11), with no difference in the Ca2+ transient time course. Diastolic stress was increased (p = 0.008) in trabeculae from diabetic patients, and peak developed stress decreased (p ≤ 0.001), which were not accounted for by reduction in the cardiomyocyte, or contractile protein, content of trabeculae. Trabeculae from diabetic patients also displayed diminished myofilament Ca2+ sensitivity (p = 0.018) compared to non-diabetic patients. Our data provides evidence of impaired calcium handling during excitation-contraction coupling with resulting contractile dysfunction in atrial tissue from patients with type 2 diabetes in comparison to the non-diabetic. This highlights the importance of targeting cardiomyocyte Ca2+ homeostasis in developing more effective treatment options for diabetic heart disease in the future.
Keywords: calcium handling; diabetes; human right atrial trabeculae; myofilament sensitivity.
© 2023 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Akella, A. B. , Ding, X. L. , Cheng, R. , & Gulati, J. (1995). Diminished Ca2+ sensitivity of skinned cardiac muscle contractility coincident with troponin T‐band shifts in the diabetic rat. Circulation Research, 76(4), 600–606. - PubMed
-
- Allo, S. N. , Lincoln, T. M. , Wilson, G. L. , Green, F. J. , Watanabe, A. M. , & Schaffer, S. W. (1991). Non‐insulin‐dependent diabetes‐induced defects in cardiac cellular calcium regulation. The American Journal of Physiology, 260(6 Pt 1), C1165–C1171. - PubMed
-
- Belke, D. D. , & Dillmann, W. H. (2004). Altered cardiac calcium handling in diabetes. Current Hypertension Reports, 6(6), 424–429. - PubMed
-
- Bers, D. M. (2002). Cardiac excitation–contraction coupling. Nature, 415(6868), 198–205. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

