Advance consent for participation in randomised controlled trials for emergency conditions: a scoping review
- PMID: 36750278
- PMCID: PMC9906254
- DOI: 10.1136/bmjopen-2022-066742
Advance consent for participation in randomised controlled trials for emergency conditions: a scoping review
Abstract
Objective: Advance consent is a recognised method of obtaining informed consent for participation in research, whereby a potential participant provides consent for future involvement in a study contingent on qualifying for the study's inclusion criteria on a later date. The goal of this study is to map the existing literature on the use of advance consent for enrolment in randomised controlled trials (RCTs) for emergency conditions.
Design: Scoping review designed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses-Extension for Scoping Reviews guidelines.
Data sources: We searched electronic databases including MEDLINE, Embase, Web of Science and the Cochrane Register of Clinical Trials from inception to 10 February 2020.
Eligibility criteria: Eligible studies included articles that discussed or employed the use of advance consent for enrolment in RCTs related to emergency conditions. There were no restrictions on the type of eligible study. Data were extracted directly from included papers using a standardised data charting form. We produced a narrative review including article type and authors' dispositions towards advance consent.
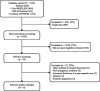
Results: Our search yielded 1039 titles with duplicates removed. Six articles met inclusion criteria. Three articles discussed the theoretical use of research advance directives in emergency conditions; one article evaluated stakeholders' perceptions of advance consent; and one article described a method for patients to document their preferences for participation in future research. Only one study employed advance consent to enrol participants into a clinical trial for an emergency condition.
Conclusion: Our review demonstrates that there has been minimal exploration of advance consent for enrolment in RCTs for emergency conditions. Future studies could aim to assess the acceptability of advance consent to participants, along with the feasibility of enrolling research participants using this method of consent.
Protocol: The protocol for this scoping review was published a priori.
Keywords: ACCIDENT & EMERGENCY MEDICINE; MEDICAL ETHICS; NEUROLOGY.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous