Rapid and Chronic Ethanol Tolerance Are Composed of Distinct Memory-Like States in Drosophila
- PMID: 36750369
- PMCID: PMC10039739
- DOI: 10.1523/JNEUROSCI.1348-22.2023
Rapid and Chronic Ethanol Tolerance Are Composed of Distinct Memory-Like States in Drosophila
Abstract
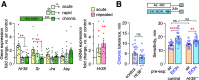
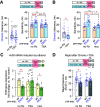
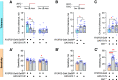
Ethanol tolerance is the first type of behavioral plasticity and neural plasticity that is induced by ethanol intake, and yet its molecular and circuit bases remain largely unexplored. Here, we characterize the following three distinct forms of ethanol tolerance in male Drosophila: rapid, chronic, and repeated. Rapid tolerance is composed of two short-lived memory-like states, one that is labile and one that is consolidated. Chronic tolerance, induced by continuous exposure, lasts for 2 d, induces ethanol preference, and hinders the development of rapid tolerance through the activity of histone deacetylases (HDACs). Unlike rapid tolerance, chronic tolerance is independent of the immediate early gene Hr38/Nr4a Chronic tolerance is suppressed by the sirtuin HDAC Sirt1, whereas rapid tolerance is enhanced by Sirt1 Moreover, rapid and chronic tolerance map to anatomically distinct regions of the mushroom body learning and memory centers. Chronic tolerance, like long-term memory, is dependent on new protein synthesis and it induces the kayak/c-fos immediate early gene, but it depends on CREB signaling outside the mushroom bodies, and it does not require the Radish GTPase. Thus, chronic ethanol exposure creates an ethanol-specific memory-like state that is molecularly and anatomically different from other forms of ethanol tolerance.SIGNIFICANCE STATEMENT The pattern and concentration of initial ethanol exposure causes operationally distinct types of ethanol tolerance to form. We identify separate molecular and neural circuit mechanisms for two forms of ethanol tolerance, rapid and chronic. We also discover that chronic tolerance forms an ethanol-specific long-term memory-like state that localizes to learning and memory circuits, but it is different from appetitive and aversive long-term memories. By contrast, rapid tolerance is composed of labile and consolidated short-term memory-like states. The multiple forms of ethanol memory-like states are genetically tractable for understanding how initial forms of ethanol-induced neural plasticity form a substrate for the longer-term brain changes associated with alcohol use disorder.
Keywords: Drosophila; alcohol; ethanol; genetics; memory; tolerance.
Copyright © 2023 the authors.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
