Rates of Status Epilepticus and Sudden Unexplained Death in Epilepsy in People With Genetic Developmental and Epileptic Encephalopathies
- PMID: 36750385
- PMCID: PMC10115508
- DOI: 10.1212/WNL.0000000000207080
Rates of Status Epilepticus and Sudden Unexplained Death in Epilepsy in People With Genetic Developmental and Epileptic Encephalopathies
Abstract
Background and objectives: The genetic developmental and epileptic encephalopathies (DEEs) comprise a large group of severe epilepsy syndromes, with a wide phenotypic spectrum. Currently, the rates of convulsive status epilepticus (CSE), nonconvulsive status epilepticus (NCSE), and sudden unexplained death in epilepsy (SUDEP) in these diseases are not well understood. We aimed to describe the proportions of patients with frequently observed genetic DEEs who developed CSE, NCSE, mortality, and SUDEP. Understanding the risks of these serious presentations in each genetic DEE will enable earlier diagnosis and appropriate management.
Methods: In this retrospective analysis of patients with a genetic DEE, we estimated the proportions with CSE, NCSE, and SUDEP and the overall and SUDEP-specific mortality rates for each genetic diagnosis. We included patients with a pathogenic variant in the genes SCN1A, SCN2A, SCN8A, SYNGAP1, NEXMIF, CHD2, PCDH19, STXBP1, GRIN2A, KCNT1, and KCNQ2 and with Angelman syndrome (AS).
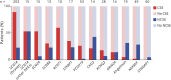
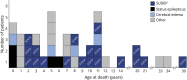
Results: The cohort comprised 510 individuals with a genetic DEE, in whom we observed CSE in 47% and NCSE in 19%. The highest proportion of CSE occurred in patients with SCN1A-associated DEEs, including 181/203 (89%; 95% CI 84-93) patients with Dravet syndrome and 8/15 (53%; 95% CI 27-79) non-Dravet SCN1A-DEEs. CSE was also notable in patients with pathogenic variants in KCNT1 (6/10; 60%; 95% CI 26-88) and SCN2A (8/15; 53%; 95% CI 27-79). NCSE was common in patients with non-Dravet SCN1A-DEEs (8/15; 53%; 95% CI 27-79) and was notable in patients with CHD2-DEEs (6/14; 43%; 95% CI 18-71) and AS (6/19; 32%; 95% CI 13-57). There were 42/510 (8%) deaths among the cohort, producing a mortality rate of 6.1 per 1,000 person-years (95% CI 4.4-8.3). Cases of SUDEP accounted for 19/42 (48%) deaths. Four genes were associated with SUDEP: SCN1A, SCN2A, SCN8A, and STXBP1. The estimated SUDEP rate was 2.8 per 1,000 person-years (95% CI 1.6-4.3).
Discussion: We showed that proportions of patients with CSE, NCSE, and SUDEP differ for commonly encountered genetic DEEs. The estimates for each genetic DEE studied will inform early diagnosis and management of status epilepticus and SUDEP and inform disease-specific counseling for patients and families in this high-risk group of conditions.
© 2023 American Academy of Neurology.
Conflict of interest statement
I.E. Scheffer has served on scientific advisory boards for BioMarin, Chiesi, Eisai, Encoded Therapeutics, GlaxoSmithKline, Knopp Biosciences, Nutricia, Rogcon, Takeda Pharmaceuticals, UCB, and Xenon Pharmaceuticals; has received speaker honoraria from GlaxoSmithKline, UCB, BioMarin, Biocodex, Chiesi, Liva Nova, Nutricia, Zuellig Pharma, and Eisai; has received funding for travel from UCB, Biocodex, GlaxoSmithKline, Biomarin, and Eisai; has served as an investigator for Anavex Life Sciences, Cerecin Inc, Cerevel Therapeutics, Eisai, Encoded Therapeutics, EpiMinder Inc, Epygenyx, ES-Therapeutics, GW Pharma, Marinus, Neurocrine BioSciences, Ovid Therapeutics, Takeda Pharmaceuticals, UCB, Ultragenyx, Xenon Pharmaceuticals, Zogenix, and Zynerba; has consulted for Care Beyond Diagnosis, Epilepsy Consortium, Atheneum Partners, Ovid Therapeutics, UCB, Zynerba Pharmaceuticals, BioMarin, Encoded Therapeutics, and Biohaven Pharmaceuticals; and is a Nonexecutive Director of Bellberry Ltd and a Director of the Australian Academy of Health and Medical Sciences and the Australian Council of Learned Academies Limited. She may accrue future revenue on pending patent WO61/010176 (filed: 2008): Therapeutic Compound; has a patent for SCN1A testing held by Bionomics Inc and licensed to various diagnostic companies; and has a patent molecular diagnostic/theranostic target for benign familial infantile epilepsy (BFIE) (PRRT2) 2011904493 & 2012900190 and PCT/AU2012/001321 (TECH ID:2012-009). The remaining authors do not have any disclosures. Go to
Figures
Comment in
-
Intellectual Disability and Epilepsy: The High Incidence and the Risks of Status Epilepticus and Sudden Death Require Improved Therapies.Epilepsy Curr. 2023 Nov 30;23(6):354-356. doi: 10.1177/15357597231203079. eCollection 2023 Nov-Dec. Epilepsy Curr. 2023. PMID: 38269346 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
