Efficacy and Safety of Tirzepatide in Type 2 Diabetes and Obesity Management
- PMID: 36750526
- PMCID: PMC10088547
- DOI: 10.7570/jomes22067
Efficacy and Safety of Tirzepatide in Type 2 Diabetes and Obesity Management
Abstract
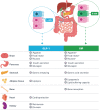
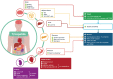
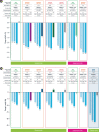
The combination of glucagon-like peptide-1 (GLP-1) with other gut hormones including the glucose-dependent insulinotropic polypeptide (GIP) has been explored to complement and enhance further the GLP-1 effects on glycemia and weight loss. Tirzepatide is the first dual GLP-1/GIP receptor co-agonist which has been approved for treatment of type 2 diabetes mellitus (T2DM) based on the findings from the SURPASS program. The SURPASS trials assessed the safety and efficacy of tirzepatide in people with T2DM, from monotherapy through to insulin add-on in global populations, with another two trials dedicated to Japanese population. Over periods of treatment up to 104 weeks, once weekly tirzepatide 5 to 15 mg reduced glycosylated hemoglobin (1.87% to 3.02%), body weight (5.4 to 12.9 kg) and improved multiple cardiometabolic risk factors (including reduction in liver fat, new-onset macroalbuminuria, blood pressure, and lipids) across the T2DM spectrum. Tirzepatide provided better efficacy than placebo and other commonly used glucose-lowering medications such as semaglutide 1 mg, dulaglutide, insulin degludec, and glargine. All tirzepatide doses were well tolerated with similar side-effect profile to the GLP-1 receptor analogues. In people without diabetes, tirzepatide 5 to 15 mg once weekly for the treatment for obesity (SURMOUNT-1) resulted in substantial reductions in body weight (16.5% to 22.4%) over 72 weeks. Overall, the SURPASS program and SURMOUNT-1 study suggest that tirzepatide is marking a new era in T2DM and/or obesity management through dual agonism of gut hormones.
Keywords: Diabetes mellitus; Gastric inhibitory polypeptide; Glucagon-like peptide 1; Obesity; Tirzepatide; type 2.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

