Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001)
- PMID: 36750721
- PMCID: PMC9905571
- DOI: 10.1038/s41392-022-01235-0
Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001)
Abstract
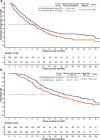
There is considerable potential for integrating transarterial chemoembolization (TACE), programmed death-(ligand)1 (PD-[L]1) inhibitors, and molecular targeted treatments (MTT) in hepatocellular carcinoma (HCC). It is necessary to investigate the therapeutic efficacy and safety of TACE combined with PD-(L)1 inhibitors and MTT in real-world situations. In this nationwide, retrospective, cohort study, 826 HCC patients receiving either TACE plus PD-(L)1 blockades and MTT (combination group, n = 376) or TACE monotherapy (monotherapy group, n = 450) were included from January 2018 to May 2021. The primary endpoint was progression-free survival (PFS) according to modified RECIST. The secondary outcomes included overall survival (OS), objective response rate (ORR), and safety. We performed propensity score matching approaches to reduce bias between two groups. After matching, 228 pairs were included with a predominantly advanced disease population. Median PFS in combination group was 9.5 months (95% confidence interval [CI], 8.4-11.0) versus 8.0 months (95% CI, 6.6-9.5) (adjusted hazard ratio [HR], 0.70, P = 0.002). OS and ORR were also significantly higher in combination group (median OS, 19.2 [16.1-27.3] vs. 15.7 months [13.0-20.2]; adjusted HR, 0.63, P = 0.001; ORR, 60.1% vs. 32.0%; P < 0.001). Grade 3/4 adverse events were observed at a rate of 15.8% and 7.5% in combination and monotherapy groups, respectively. Our results suggest that TACE plus PD-(L)1 blockades and MTT could significantly improve PFS, OS, and ORR versus TACE monotherapy for Chinese patients with predominantly advanced HCC in real-world practice, with an acceptable safety profile.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

