Potential molecular mechanisms of chronic fatigue in long haul COVID and other viral diseases
- PMID: 36750846
- PMCID: PMC9902840
- DOI: 10.1186/s13027-023-00485-z
Potential molecular mechanisms of chronic fatigue in long haul COVID and other viral diseases
Erratum in
-
Correction: Potential molecular mechanisms of chronic fatigue in long haul COVID and other viral diseases.Infect Agent Cancer. 2023 Apr 24;18(1):23. doi: 10.1186/s13027-023-00505-y. Infect Agent Cancer. 2023. PMID: 37095550 Free PMC article. No abstract available.
Abstract
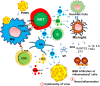
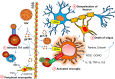
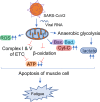
Historically, COVID-19 emerges as one of the most devastating diseases of humankind, which creates an unmanageable health crisis worldwide. Until now, this disease costs millions of lives and continues to paralyze human civilization's economy and social growth, leaving an enduring damage that will take an exceptionally long time to repair. While a majority of infected patients survive after mild to moderate reactions after two to six weeks, a growing population of patients suffers for months with severe and prolonged symptoms of fatigue, depression, and anxiety. These patients are no less than 10% of total COVID-19 infected individuals with distinctive chronic clinical symptomatology, collectively termed post-acute sequelae of COVID-19 (PASC) or more commonly long-haul COVID. Interestingly, Long-haul COVID and many debilitating viral diseases display a similar range of clinical symptoms of muscle fatigue, dizziness, depression, and chronic inflammation. In our current hypothesis-driven review article, we attempt to discuss the molecular mechanism of muscle fatigue in long-haul COVID, and other viral diseases as caused by HHV6, Powassan, Epstein-Barr virus (EBV), and HIV. We also discuss the pathological resemblance of virus-triggered muscle fatigue with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
Keywords: CD4+ and CD8+ T cells; IFNγ; Microglia; Mitochondria.
© 2023. The Author(s).
Conflict of interest statement
AR, GG, JA, and DP are employee of Simmaron Research INC, a 501C non-profit research organization. All authors declare no competing interests.
Figures



Similar articles
-
Orthostatic Symptoms and Reductions in Cerebral Blood Flow in Long-Haul COVID-19 Patients: Similarities with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome.Medicina (Kaunas). 2021 Dec 24;58(1):28. doi: 10.3390/medicina58010028. Medicina (Kaunas). 2021. PMID: 35056336 Free PMC article.
-
Saliva antibody-fingerprint of reactivated latent viruses after mild/asymptomatic COVID-19 is unique in patients with myalgic-encephalomyelitis/chronic fatigue syndrome.Front Immunol. 2022 Oct 20;13:949787. doi: 10.3389/fimmu.2022.949787. eCollection 2022. Front Immunol. 2022. PMID: 36341457 Free PMC article.
-
Orthostatic Intolerance in Long-Haul COVID after SARS-CoV-2: A Case-Control Comparison with Post-EBV and Insidious-Onset Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients.Healthcare (Basel). 2022 Oct 17;10(10):2058. doi: 10.3390/healthcare10102058. Healthcare (Basel). 2022. PMID: 36292504 Free PMC article.
-
Persistent SARS-CoV-2 Infection, EBV, HHV-6 and Other Factors May Contribute to Inflammation and Autoimmunity in Long COVID.Viruses. 2023 Jan 31;15(2):400. doi: 10.3390/v15020400. Viruses. 2023. PMID: 36851614 Free PMC article. Review.
-
Insights from myalgic encephalomyelitis/chronic fatigue syndrome may help unravel the pathogenesis of postacute COVID-19 syndrome.Trends Mol Med. 2021 Sep;27(9):895-906. doi: 10.1016/j.molmed.2021.06.002. Epub 2021 Jun 7. Trends Mol Med. 2021. PMID: 34175230 Free PMC article. Review.
Cited by
-
Revealing the Complexity of Fatigue: A Review of the Persistent Challenges and Promises of Artificial Intelligence.Brain Sci. 2024 Feb 19;14(2):186. doi: 10.3390/brainsci14020186. Brain Sci. 2024. PMID: 38391760 Free PMC article. Review.
-
Repurposing Licensed Drugs with Activity Against Epstein-Barr Virus for Treatment of Multiple Sclerosis: A Systematic Approach.CNS Drugs. 2025 Mar;39(3):305-320. doi: 10.1007/s40263-024-01153-5. Epub 2025 Jan 10. CNS Drugs. 2025. PMID: 39792343
-
Symptom Clusters in Acute SARS-CoV-2 Infection and Long COVID Fatigue in Male and Female Outpatients.J Pers Med. 2024 Jun 5;14(6):602. doi: 10.3390/jpm14060602. J Pers Med. 2024. PMID: 38929823 Free PMC article.
-
Multimodal Telerehabilitation in Post COVID-19 Condition Recovery: A Series of 12 Cases.Reports (MDPI). 2025 Mar 20;8(1):35. doi: 10.3390/reports8010035. Reports (MDPI). 2025. PMID: 40729248 Free PMC article.
-
The demographic, laboratory and genetic factors associated with long Covid-19 syndrome: a case-control study.Clin Exp Med. 2024 Jan 17;24(1):1. doi: 10.1007/s10238-023-01256-1. Clin Exp Med. 2024. PMID: 38231284 Free PMC article.
References
-
- Bateman L, Bested AC, Bonilla HF, Chheda BV, Chu L, Curtin JM, Dempsey TT, Dimmock ME, Dowell TG, Felsenstein D, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: essentials of diagnosis and management. Mayo Clin Proc. 2021;96(11):2861–2878. - PubMed
-
- VanNess JM, Stevens SR, Bateman L, Stiles TL, Snell CR. Postexertional malaise in women with chronic fatigue syndrome. J Womens Health (Larchmt) 2010;19(2):239–244. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

