Korean Practice Guidelines for Gastric Cancer 2022: An Evidence-based, Multidisciplinary Approach
- PMID: 36750993
- PMCID: PMC9911619
- DOI: 10.5230/jgc.2023.23.e11
Korean Practice Guidelines for Gastric Cancer 2022: An Evidence-based, Multidisciplinary Approach
Erratum in
-
Erratum: Korean Practice Guidelines for Gastric Cancer 2022: An Evidence-based, Multidisciplinary Approach.J Gastric Cancer. 2023 Apr;23(2):365-373. doi: 10.5230/jgc.2023.23.e20. J Gastric Cancer. 2023. PMID: 37129159 Free PMC article.
Abstract
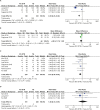
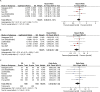
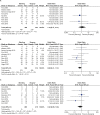
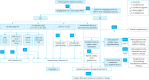
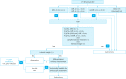
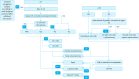
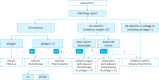
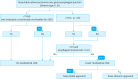
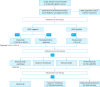
Gastric cancer is one of the most common cancers in Korea and the world. Since 2004, this is the 4th gastric cancer guideline published in Korea which is the revised version of previous evidence-based approach in 2018. Current guideline is a collaborative work of the interdisciplinary working group including experts in the field of gastric surgery, gastroenterology, endoscopy, medical oncology, abdominal radiology, pathology, nuclear medicine, radiation oncology and guideline development methodology. Total of 33 key questions were updated or proposed after a collaborative review by the working group and 40 statements were developed according to the systematic review using the MEDLINE, Embase, Cochrane Library and KoreaMed database. The level of evidence and the grading of recommendations were categorized according to the Grading of Recommendations, Assessment, Development and Evaluation proposition. Evidence level, benefit, harm, and clinical applicability was considered as the significant factors for recommendation. The working group reviewed recommendations and discussed for consensus. In the earlier part, general consideration discusses screening, diagnosis and staging of endoscopy, pathology, radiology, and nuclear medicine. Flowchart is depicted with statements which is supported by meta-analysis and references. Since clinical trial and systematic review was not suitable for postoperative oncologic and nutritional follow-up, working group agreed to conduct a nationwide survey investigating the clinical practice of all tertiary or general hospitals in Korea. The purpose of this survey was to provide baseline information on follow up. Herein we present a multidisciplinary-evidence based gastric cancer guideline.
Keywords: Chemotherapy; Endoscopy; Guidelines; Stomach neoplasms; Surgery.
Copyright © 2023. Korean Gastric Cancer Association.
Conflict of interest statement
Seong-Ho Kong has received research funding from Stryker Co., Ltd. and Medtronic Inc. as the principal investigator and is the CEO of VITCAL, Co., Ltd. No potential conflict of interest relevant to this article was reported.
Figures




















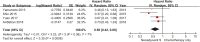


Comment in
-
The Necessity of Guidance: Optimizing Adjuvant Therapy for Stage II/III MSI-H Gastric Cancer Through the Interplay of Evidence, Clinical Judgment, and Patient Preferences.J Gastric Cancer. 2024 Jul;24(3):243-245. doi: 10.5230/jgc.2024.24.e26. J Gastric Cancer. 2024. PMID: 38960883 Free PMC article. No abstract available.
References
-
- International Agency for Research on Cancer. Cancer fact sheet stomach: Globocan 2020 [Internet] Geneva: World Health Organization; 2022. Available from: https://gco.iarc.fr/today/data/factsheets/cancers/7-Stomach-fact-sheet.pdf.
-
- National Cancer Center, Korea Central Cancer Registry. Korean Cancer Report 2020. Goyang: National Cancer Center; 2022.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

