Renal Disease in Cryoglobulinemia
- PMID: 36751424
- PMCID: PMC9677724
- DOI: 10.1159/000516103
Renal Disease in Cryoglobulinemia
Abstract
Background: Renal disease in cryoglobulinemia is difficult to grasp and diagnose because it is rare, serological testing is challenging and prone to artifacts, and its morphology is shared by other renal diseases resulting in a spectrum of differential diagnoses. On occasion, a definitive diagnosis cannot even be rendered after immunofluorescence and electron microscopic studies.
Summary: Based on kidney biopsies seen in our routine diagnostic and referral practice, we discuss and illustrate various morphological patterns of renal injury associated with cryoglobulins. We outline key pathophysiologic and clinical aspects associated with cryoglobulinemia induced renal disease and describe morphologic changes with a focus on electron microscopy. We present our practical, morphology-based approach to diagnostic decision-making with special consideration of differential diagnoses and disease mimickers. Since cryoglobulins are rarely tested for prior to kidney biopsy, pathologists and clinicians alike must have a high level of suspicion when interpreting renal biopsies and managing patients.
Key messages: Cryoglobulinemia-associated glomerulonephritis (GN) is a multifactorial disease which is important to recognize for clinical practice. Morphological features suggestive of cryoglobulinemia-associated GN include a pattern of membranoproliferative GN with abundance of monocytes and the presence of (pseudo)thrombi. By electron microscopy, the main diagnostic features are a prominent infiltration of monocytes/macrophages and the presence of mesangial and subendothelial deposits with frequently curved microtubular/cylindrical and annular substructures.
Keywords: Electron microscopy; Glomerulonephritis; Hepatitis C; Structured deposits.
Copyright © 2021 by The Author(s) Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
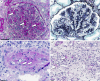
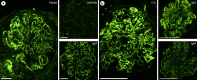
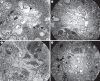
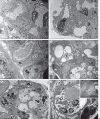

References
-
- Basile U, Torti E, Dell'Abate MT, Colacicco L, Gulli F, Zuppi C, et al. Pre-analytical phase in cryoglobulin detection: an alternative method for sample transport. Clin Chem Lab Med. 2016;54:e123–e126. - PubMed
-
- Brouet JC, Clauvel JP, Danon F, Klein M, Seligmann M. Biologic and clinical significance of cryoglobulins. A report of 86 cases. Am J Med. 1974;57((5)):775–788. - PubMed
-
- Kolopp-Sarda MN, Nombel A, Miossec P. Cryoglobulins today: detection and immunologic characteristics of 1,675 positive samples from 13,439 patients obtained over six years. Arthritis Rheumatol. 2019;71((11)):1904–1912. - PubMed
-
- Roccatello D, Saadoun D, Ramos-Casals M, Tzioufas AG, Fervenza FC, Cacoub P, et al. Cryoglobulinaemia. Nat Rev Dis Primers. 2018;4((1)):11. - PubMed
-
- Strait RT, Posgai MT, Mahler A, Barasa N, Jacob CO, Köhl J, et al. Erratum: IgG1 protects against renal disease in a mouse model of cryoglobulinaemia. Nature. 2015;526((7575)):728–724. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
