Comparing the efficacy and safety of IL-17 inhibitors for treatment of moderate-to-severe psoriasis: a randomized double blind pilot study with a review of literature
- PMID: 36751540
- PMCID: PMC9880774
- DOI: 10.5114/ada.2019.91496
Comparing the efficacy and safety of IL-17 inhibitors for treatment of moderate-to-severe psoriasis: a randomized double blind pilot study with a review of literature
Abstract
Introduction: Psoriasis is a chronic skin disease in which interleukin-17A (IL-17A) has been found to play an important role. Commercially available anti-IL-17 drugs include brodalumab, ixekizumab (IXE), and secukinumab (SEC).
Aim: To compare the safety and efficacy of IXE and SEC in patients with moderate-to-severe plaque psoriasis.
Material and methods: The patients were randomized to the IXE or SEC group. Effectiveness was estimated by Physician's Global Assessment (PGA), Psoriasis Area and Severity Index (PASI), and Dermatology Life Quality Index (DLQI). Safety was assessed by documentation of adverse effects (AEs), routine laboratory values, and injection-site and allergic reactions.
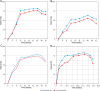
Results: There were 155 patients in the IXE group and 158 in the SEC group. At week 12, PASI 75 was 76.77% (IXE) vs. 67.09% (SEC); PASI 90 42.58% (IXE) vs. 32.28% (SEC); PGA score of 0 or 1 at week 40 (79.52% vs. 74.4%) and at week 52 (61.83% vs. 58.12%) (p < 0.001). Also, DLQI score improvement was more pronounced in the IXE group. The rates and types of AEs were similar in both groups.
Conclusions: Although both groups demonstrated a robust clinical response, a significant improvement in patient quality of life, and a satisfactory safety profile, the IXE group scored a notch higher.
Keywords: IL-17 inhibitors; ixekizumab; plaque psoriasis; secukinumab.
Copyright © 2022 Termedia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med 2009; 361: 496-509. - PubMed
-
- Chon MP, Boehncke WH. Psoriasis. N Engl J Med 2005; 352: 1899-912. - PubMed
-
- Griffiths CEM, Barker JNWN. Pathogenesis and clinical features of psoriasis. Lancet 2007; 370: 263-71. - PubMed
-
- Koo J. Population-based epidemiologic study of psoriasis with emphasis on quality of life assessment. Dermatol Clin 1996; 14: 485-96. - PubMed
LinkOut - more resources
Full Text Sources