Death-associated protein kinase 1 is associated with cognitive dysfunction in major depressive disorder
- PMID: 36751808
- PMCID: PMC10154471
- DOI: 10.4103/1673-5374.361532
Death-associated protein kinase 1 is associated with cognitive dysfunction in major depressive disorder
Abstract
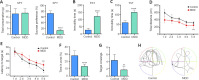
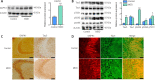
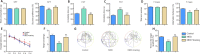
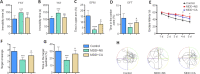
We previously showed that death-associated protein kinase 1 (DAPK1) expression is increased in hippocampal tissue in a mouse model of major depressive disorde and is related to cognitive dysfunction in Alzheimer's disease. In addition, depression is a risk factor for developing Alzheimer's disease, as well as an early clinical manifestation of Alzheimer's disease. Meanwhile, cognitive dysfunction is a distinctive feature of major depressive disorder. Therefore, DAPK1 may be related to cognitive dysfunction in major depressive disorder. In this study, we established a mouse model of major depressive disorder by housing mice individually and exposing them to chronic, mild, unpredictable stressors. We found that DAPK1 and tau protein levels were increased in the hippocampal CA3 area, and tau was hyperphosphorylated at Thr231, Ser262, and Ser396 in these mice. Furthermore, DAPK1 shifted from axonal expression to overexpression on the cell membrane. Exercise and treatment with the antidepressant drug citalopram decreased DAPK1 expression and tau protein phosphorylation in hippocampal tissue and improved both depressive symptoms and cognitive dysfunction. These results indicate that DAPK1 may be a potential reason and therapeutic target of cognitive dysfunction in major depressive disorder.
Keywords: Alzheimer’s disease; antidepressant drug; behavioral tests; cognitive dysfunction; death-associated protein kinase 1; exercise; hippocampus; major depressive disorder; phosphorylation; tau protein.
Conflict of interest statement
None
Figures


Similar articles
-
Phosphorylation of tau by death-associated protein kinase 1 antagonizes the kinase-induced cell apoptosis.J Alzheimers Dis. 2013;37(4):795-808. doi: 10.3233/JAD-130377. J Alzheimers Dis. 2013. PMID: 23948915
-
miR-143-3p Inhibits Aberrant Tau Phosphorylation and Amyloidogenic Processing of APP by Directly Targeting DAPK1 in Alzheimer's Disease.Int J Mol Sci. 2022 Jul 20;23(14):7992. doi: 10.3390/ijms23147992. Int J Mol Sci. 2022. PMID: 35887339 Free PMC article.
-
Death-associated protein kinase 1 has a critical role in aberrant tau protein regulation and function.Cell Death Dis. 2014 May 22;5(5):e1237. doi: 10.1038/cddis.2014.216. Cell Death Dis. 2014. PMID: 24853415 Free PMC article.
-
Death-Associated Protein Kinase 1 as a Promising Drug Target in Cancer and Alzheimer's Disease.Recent Pat Anticancer Drug Discov. 2019;14(2):144-157. doi: 10.2174/1574892814666181218170257. Recent Pat Anticancer Drug Discov. 2019. PMID: 30569876 Free PMC article. Review.
-
DAPK1: a Novel Pathology and Treatment Target for Alzheimer's Disease.Mol Neurobiol. 2019 Apr;56(4):2838-2844. doi: 10.1007/s12035-018-1242-2. Epub 2018 Jul 31. Mol Neurobiol. 2019. PMID: 30062675 Review.
Cited by
-
Death-associated protein kinase 1 as a therapeutic target for Alzheimer's disease.Transl Neurodegener. 2024 Jan 9;13(1):4. doi: 10.1186/s40035-023-00395-5. Transl Neurodegener. 2024. PMID: 38195518 Free PMC article. Review.
-
Ventral tegmental area dopaminergic circuits participates in stress-induced chronic postsurgical pain in male mice.BMC Neurosci. 2024 Jan 9;25(1):3. doi: 10.1186/s12868-023-00842-z. BMC Neurosci. 2024. PMID: 38195391 Free PMC article.
-
Peripheral mitochondrial DNA as a neuroinflammatory biomarker for major depressive disorder.Neural Regen Res. 2025 Jun 1;20(6):1541-1554. doi: 10.4103/NRR.NRR-D-23-01878. Epub 2024 Jun 26. Neural Regen Res. 2025. PMID: 38934398 Free PMC article.
-
Acanthopanax Senticosus Saponins Prevent Cognitive Decline in Rats with Alzheimer's Disease.Int J Mol Sci. 2025 Apr 14;26(8):3715. doi: 10.3390/ijms26083715. Int J Mol Sci. 2025. PMID: 40332373 Free PMC article.
-
End-stage renal disease accompanied by mild cognitive impairment: A study and analysis of trimodal brain network fusion.PLoS One. 2024 Jun 13;19(6):e0305079. doi: 10.1371/journal.pone.0305079. eCollection 2024. PLoS One. 2024. PMID: 38870175 Free PMC article.
References
-
- Abraham EH, Khan B, Ling E, Bernstein LJ. The development and evaluation of a patient educational resource for cancer-related cognitive dysfunction. J Cancer Educ. (2022);37:111–119. - PubMed
-
- Agnihotri A, Aruoma OI. Alzheimer's disease and Parkinson's disease:a nutritional toxicology perspective of the impact of oxidative stress, mitochondrial, dysfunction, nutrigenomics, and environmental chemicals. J Am Coll Nutr. (2020);39:16–27. - PubMed
-
- Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia:a population-based perspective. Alzheimers Dement. (2015);11:718–726. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

