OnabotulinumtoxinA effects on trigeminal nociceptors
- PMID: 36751871
- PMCID: PMC10652784
- DOI: 10.1177/03331024221141683
OnabotulinumtoxinA effects on trigeminal nociceptors
Abstract
Background: OnabotulinumtoxinA (onabotA) is approved globally for prevention of chronic migraine; however, the classical mechanism of action of onabotA in motor and autonomic neurons cannot fully explain the effectiveness of onabotulinumtoxinA in this sensory neurological disease. We sought to explore the direct effects of onabotulinumtoxinA on mouse trigeminal ganglion sensory neurons using an inflammatory soup-based model of sensitization.
Methods: Primary cultured trigeminal ganglion neurons were pre-treated with inflammatory soup, then treated with onabotulinumtoxinA (2.75 pM). Treated neurons were used to examine transient receptor potential vanilloid subtype 1 and transient receptor potential ankyrin 1 cell-surface expression, calcium influx, and neuropeptide release.
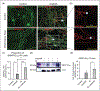
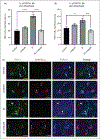
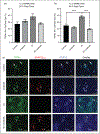
Results: We found that onabotulinumtoxinA cleaved synaptosomal-associated protein-25 kDa in cultured trigeminal ganglion neurons; synaptosomal-associated protein-25 kDa cleavage was enhanced by inflammatory soup pre-treatment, suggesting greater uptake of toxin under sensitized conditions. OnabotulinumtoxinA also prevented inflammatory soup-mediated increases in TRPV1 and TRPA1 cell-surface expression, without significantly altering TRPV1 or TRPA1 protein expression in unsensitized conditions. We observed similar inhibitory effects of onabotulinumtoxinA on TRP-mediated calcium influx and TRPV1- and TRPA1-mediated release of calcitonin gene-related peptide and prostaglandin 2 under sensitized, but not unsensitized control, conditions.
Conclusions: Our data deepen the understanding of the sensory mechanism of action of onabotulinumtoxinA and support the notion that, once endocytosed, the cytosolic light chain of onabotulinumtoxinA cleaves synaptosomal-associated protein-25 kDa to prevent soluble N-ethylmaleimide-sensitive factor attachment protein receptor-mediated processes more generally in motor, autonomic, and sensory neurons.
Keywords: Inflammatory soup; botulinum neurotoxin type A; chronic migraine; transient receptor potential ankyrin 1 (TRPA1); transient receptor potential vanilloid subtype 1 (TRPV1); trigeminal ganglion.
Conflict of interest statement
Declaration of conflicting interests
AAM, CW, SC, GM, EX, and LO have no conflicts of interest to disclose.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

