Baseline characteristics of the North American prodromal Synucleinopathy cohort
- PMID: 36751940
- PMCID: PMC10109527
- DOI: 10.1002/acn3.51738
Baseline characteristics of the North American prodromal Synucleinopathy cohort
Abstract
Objective: Rapid eye movement (REM) sleep behavior disorder (RBD) is widely considered a prodromal synucleinopathy, as most with RBD develop overt synucleinopathy within ~10 years. Accordingly, RBD offers an opportunity to test potential treatments at the earliest stages of synucleinopathy. The North American Prodromal Synucleinopathy (NAPS) Consortium has created a multisite RBD participant, primarily clinic-based cohort to better understand characteristics at diagnosis, and in future work, identify predictors of phenoconversion, develop synucleinopathy biomarkers, and enable early stage clinical trial enrollment.
Methods: Participants ≥18 years of age with overnight polysomnogram-confirmed RBD without Parkinson's disease, dementia, multiple system atrophy, or narcolepsy were enrolled from nine sites across North America (8/2018 to 4/2021). Data collection included family/personal history of RBD and standardized assessments of cognitive, motor, sensory, and autonomic function.
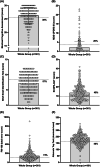
Results: Outcomes are primarily reported based on sex (361 total: n = 295 male, n = 66 female), and secondarily based on history of antidepressant use (n = 200 with, n = 154 without; with correction for sex differences) and based on extent of synucleinopathy burden (n = 56 defined as isolated RBD, n = 305 defined as RBD+ [i.e., exhibiting ≥1 abnormality]). Overall, these participants commonly demonstrated abnormalities in global cognition (MoCA; 38%), motor function (alternate tap test; 48%), sensory (BSIT; 57%), autonomic function (orthostatic hypotension, 38.8%), and anxiety/depression (BAI and PHQ-9; 39.3% and 31%, respectively).
Interpretation: These RBD participants, assessed with extensive history, demographic, cognitive, motor, sensory, and autonomic function demonstrated a lack of sex differences and high frequency of concomitant neurological abnormalities. These participants will be valuable for future longitudinal study and neuroprotective clinical trials.
© 2023 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Dr. Elliott has received support from the Department of Veteran Affairs, NIH (NHLBI, NIA, NCCIH), Oregon Medical Research Foundation, Portland VA Research Foundation, Eugene & Clarissa Evonuk Foundation in Environmental Physiology, and American Heart Association.
Dr. Lim has received support from Department of Veteran Affairs, Department of Defense, NIH (NIMH, NHLBI, NIA, NCCIH, NINDS, NIGMS, NCATS), NSF, Center for Neuroscience & Regenerative Medicine (Henry Jackson Foundation), Oregon Medical Research Foundation, Collins Medical Trust, Brain & Behavior Foundation (NARSAD), American Sleep Medicine Foundation, Hartford Center of Gerontological Excellence, Pacific Northwest National Laboratory, and Portland VA Research Foundation.
Allison Keil, Dr. Pelletier, Dr. Forsberg, Jennifer McLeland has no disclosures.
Dr. Postuma has received support from the Fonds de Recherche du Quebec – Santé, the Canadian Institutes of Health Research, the Parkinson Society of Canada, the Weston‐Garfield Foundation, the Michael J. Fox Foundation, the Webster Foundation; and personal fees from Takeda, Roche/Prothena, Teva Neurosciences, Novartis Canada, Biogen, Boehringer Ingelheim, Ther‐ anexus, GE HealthCare, Jazz Pharmaceuticals, Abbvie, Jannsen, Otsuko, Phytopharmics, Inception Sciences, and Curasen.
Dr. Gagnon has received support from the NIH, the Canadian Institutes of Health Research and the
Fonds de Recherche du Québec – Santé .Dr. St. Louis has received support from NIH (NIA, NINDS, and NHLBI), the Michael J. Fox Foundation, Harmony, Inc., and Sunovion, Inc.
Dr. Fields has received support from the NIH.
Dr. Bliwise has received support from the NIH and has been a Consultant to CliniLabs, Eisai, Ferring, Huxley, Idorsia, and Merck.
Dr. Huddleston has received support from NIH (NIA, NINDS, Department of Veteran Affairs, the American Parkinson's Disease Association Center for Advanced Research, the Emory Udall Parkinson's Disease Research Center, the Emory Lewy Body Dementia Association Research Center of Excellence, the Emory Alzheimer's Disease Research Center, the Michael J Fox Foundation, the Georgia Research Alliance, the Bumpus Family Foundation, and the McMahon Family).
Dr. Avidan has received consultant fees from Avadel, Merck, Takeda, Eisai, Idorsia, and Harmony, and speaker honoraria from Merck, Eisai, Harmony, and Idorsia.
Dr. Howell has received research support from the NIH.
Dr. Schenck has received a one‐time speaker honorarium from Eisai, Inc.
Dr. Criswell has received support from the NIH and consulting fees from Abbvie and Sio Gene Therapies.
Dr. Videnovic has received research support from the NIH and the Michael J Fox Foundation; consultancy fees from Alexion Pharmaceuticals, Biogen, XW Pharma, Jazz.
Dr. During has received support from Jazz Pharmaceuticals, Sanofi, Takeda, Rythm Inc., and the Feldman Foundation CA.
Dr. Miglis has received support from Jazz Pharmaceuticals, Embr Wave, and Biohaven Pharmaceuticals; Consulting fees from 2nd MD, Infinite MD, and Guidepoint LLC; Payments for CME lectures from MED‐IQ; Royalties from Elsevier Inc.
Dr. Shprecher has received support from the Arizona Alzheimer's Consortium, Abbvie, Acadia, Aptinyx, Axovant, Biogen, Eisai, Eli Lilly, Enterin, Neurocrine, Michael J Fox Foundation, NIH, Nuvelution, Theravance, and Teva; consultant fees from Amneal, Forensis, and Neurocrine; speaker honoraria from Acorda, Amneal, Neurocrine, Sunovion, Teva, and US World Meds/Supernus.
Dr. Lee‐Iannotti has received support from NIH, Liva Nova, Respicardia, and the Arizona Alzheimer's Consortium. She serves on the Scientific Advisory Board for Jazz Pharmaceuticals, INSPIRE, and Avadel. She is a consultant and speaker for Jazz Pharmaceuticals.
Dr. Boeve has served as an investigator for clinical trials sponsored by Alector and Biogen. He serves on the Scientific Advisory Board of the Tau Consortium. He receives support from NIH, the Mayo Clinic Dorothy, and Harry T. Mangurian Jr. Lewy Body Dementia Program, the Little Family Foundation, and the Ted Turner and Family Foundation.
Dr. Ju has received support from the NIH and the Centene Corporation contract (P19‐00559) for the Washington University‐Centene ARCH Personalized Medicine Initiative; and compensation for consultant activities for Applied Cognition.
Figures
References
-
- Kowal SL, Dall TM, Chakrabarti R, Storm MV, Jain A. The current and projected economic burden of Parkinson's disease in the United States. Mov Disord. 2013;28(3):311‐318. - PubMed
-
- Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9:293‐308. - PubMed
-
- Dauvilliers Y, Schenck CH, Postuma RB, et al. REM sleep behaviour disorder. Nat Rev Dis Prim. 2018;4(1):19. - PubMed

