Loss-of-function mutations in CFAP57 cause multiple morphological abnormalities of the flagella in humans and mice
- PMID: 36752199
- PMCID: PMC9977434
- DOI: 10.1172/jci.insight.166869
Loss-of-function mutations in CFAP57 cause multiple morphological abnormalities of the flagella in humans and mice
Abstract
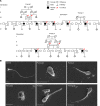
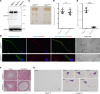
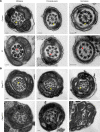
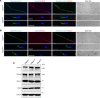
Multiple morphological abnormalities of the sperm flagella (MMAF) are the most severe form of asthenozoospermia due to impaired axoneme structure in sperm flagella. Dynein arms are necessary components of the sperm flagellar axoneme. In this study, we recruited 3 unrelated consanguineous Pakistani families with multiple MMAF-affected individuals, who had no overt ciliary symptoms. Whole-exome sequencing and Sanger sequencing identified 2 cilia and flagella associated protein 57 (CFAP57) loss-of-function mutations (c.2872C>T, p. R958*; and c.2737C>T, p. R913*) recessively segregating with male infertility. A mouse model mimicking the mutation (c.2872C>T) was generated and recapitulated the typical MMAF phenotype of CFAP57-mutated individuals. Both CFAP57 mutations caused loss of the long transcript-encoded CFAP57 protein in spermatozoa from MMAF-affected individuals or from the Cfap57-mutant mouse model while the short transcript was not affected. Subsequent examinations of the spermatozoa from Cfap57-mutant mice revealed that CFAP57 deficiency disrupted the inner dynein arm (IDA) assembly in sperm flagella and that single-headed IDAs were more likely to be affected. Thus, our study identified 2 pathogenic mutations in CFAP57 in MMAF-affected individuals and reported a conserved and pivotal role for the long transcript-encoded CFAP57 in IDAs' assembly and male fertility.
Keywords: Fertility; Genetic diseases; Mouse models; Reproductive Biology.
Figures

Similar articles
-
Mutation of CFAP57, a protein required for the asymmetric targeting of a subset of inner dynein arms in Chlamydomonas, causes primary ciliary dyskinesia.PLoS Genet. 2020 Aug 7;16(8):e1008691. doi: 10.1371/journal.pgen.1008691. eCollection 2020 Aug. PLoS Genet. 2020. PMID: 32764743 Free PMC article.
-
Whole-exome sequencing of familial cases of multiple morphological abnormalities of the sperm flagella (MMAF) reveals new DNAH1 mutations.Hum Reprod. 2016 Dec;31(12):2872-2880. doi: 10.1093/humrep/dew262. Epub 2016 Oct 26. Hum Reprod. 2016. PMID: 27798045
-
TTC12 Loss-of-Function Mutations Cause Primary Ciliary Dyskinesia and Unveil Distinct Dynein Assembly Mechanisms in Motile Cilia Versus Flagella.Am J Hum Genet. 2020 Feb 6;106(2):153-169. doi: 10.1016/j.ajhg.2019.12.010. Epub 2020 Jan 23. Am J Hum Genet. 2020. PMID: 31978331 Free PMC article.
-
Novel DNAH1 Mutation Loci Lead to Multiple Morphological Abnormalities of the Sperm Flagella and Literature Review.World J Mens Health. 2022 Oct;40(4):551-560. doi: 10.5534/wjmh.210119. Epub 2022 Jan 25. World J Mens Health. 2022. PMID: 35118838 Free PMC article. Review.
-
Molecular genetics of infertility: loss-of-function mutations in humans and corresponding knockout/mutated mice.Hum Reprod Update. 2021 Jan 4;27(1):154-189. doi: 10.1093/humupd/dmaa034. Hum Reprod Update. 2021. PMID: 33118031 Review.
Cited by
-
Homozygous CCDC146 mutation causes oligoasthenoteratozoospermia in humans and mice.Zool Res. 2024 Sep 18;45(5):1073-1087. doi: 10.24272/j.issn.2095-8137.2024.019. Zool Res. 2024. PMID: 39245651 Free PMC article.
-
Genetic Causes of Qualitative Sperm Defects: A Narrative Review of Clinical Evidence.Genes (Basel). 2024 May 8;15(5):600. doi: 10.3390/genes15050600. Genes (Basel). 2024. PMID: 38790229 Free PMC article. Review.
-
Editorial: Molecular and cytogenetic research advances in human reproduction - volume II.Front Endocrinol (Lausanne). 2023 Jul 17;14:1232953. doi: 10.3389/fendo.2023.1232953. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37529612 Free PMC article. No abstract available.
-
LRRC56 deletion causes primary ciliary dyskinesia in mice characterized by dynein arms defects.Biol Open. 2025 Feb 15;14(2):bio061846. doi: 10.1242/bio.061846. Epub 2025 Feb 5. Biol Open. 2025. PMID: 39912490 Free PMC article.
-
A novel homozygous splicing mutation in AK7 causes multiple morphological abnormalities of sperm flagella in patients from consanguineous Pakistani families.Asian J Androl. 2025 Mar 1;27(2):189-195. doi: 10.4103/aja202471. Epub 2024 Sep 10. Asian J Androl. 2025. PMID: 39254435 Free PMC article.
References
-
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. Sixth Ed. WHO; 2021. - PubMed

