Inhibition of antiapoptotic BCL-2 proteins with ABT-263 induces fibroblast apoptosis, reversing persistent pulmonary fibrosis
- PMID: 36752201
- PMCID: PMC9977433
- DOI: 10.1172/jci.insight.163762
Inhibition of antiapoptotic BCL-2 proteins with ABT-263 induces fibroblast apoptosis, reversing persistent pulmonary fibrosis
Abstract
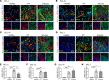
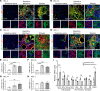
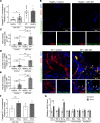
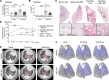
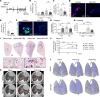
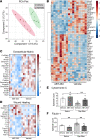
Patients with progressive fibrosing interstitial lung diseases (PF-ILDs) carry a poor prognosis and have limited therapeutic options. A hallmark feature is fibroblast resistance to apoptosis, leading to their persistence, accumulation, and excessive deposition of extracellular matrix. A complex balance of the B cell lymphoma 2 (BCL-2) protein family controlling the intrinsic pathway of apoptosis and fibroblast reliance on antiapoptotic proteins has been hypothesized to contribute to this resistant phenotype. Examination of lung tissue from patients with PF-ILD (idiopathic pulmonary fibrosis and silicosis) and mice with PF-ILD (repetitive bleomycin and silicosis) showed increased expression of antiapoptotic BCL-2 family members in α-smooth muscle actin-positive fibroblasts, suggesting that fibroblasts from fibrotic lungs may exhibit increased susceptibility to inhibition of antiapoptotic BCL-2 family members BCL-2, BCL-XL, and BCL-W with the BH3 mimetic ABT-263. We used 2 murine models of PF-ILD to test the efficacy of ABT-263 in reversing established persistent pulmonary fibrosis. Treatment with ABT-263 induced fibroblast apoptosis, decreased fibroblast numbers, and reduced lung collagen levels, radiographic disease, and histologically evident fibrosis. Our studies provide insight into how fibroblasts gain resistance to apoptosis and become sensitive to the therapeutic inhibition of antiapoptotic proteins. By targeting profibrotic fibroblasts, ABT-263 offers a promising therapeutic option for PF-ILDs.
Keywords: Apoptosis pathways; Cell Biology; Fibrosis; Mouse models; Pulmonology.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

