Notch-mediated hepatocyte MCP-1 secretion causes liver fibrosis
- PMID: 36752206
- PMCID: PMC9977430
- DOI: 10.1172/jci.insight.165369
Notch-mediated hepatocyte MCP-1 secretion causes liver fibrosis
Abstract
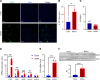
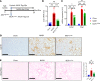
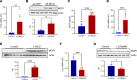
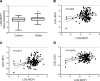
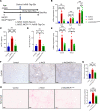
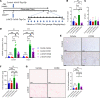
Patients with nonalcoholic steatohepatitis (NASH) have increased expression of liver monocyte chemoattractant protein-1 (MCP-1), but its cellular source and contribution to various aspects of NASH pathophysiology remain debated. We demonstrated increased liver CCL2 (which encodes MCP-1) expression in patients with NASH, and commensurately, a 100-fold increase in hepatocyte Ccl2 expression in a mouse model of NASH, accompanied by increased liver monocyte-derived macrophage (MoMF) infiltrate and liver fibrosis. To test repercussions of increased hepatocyte-derived MCP-1, we generated hepatocyte-specific Ccl2-knockout mice, which showed reduced liver MoMF infiltrate as well as decreased liver fibrosis. Forced hepatocyte MCP-1 expression provoked the opposite phenotype in chow-fed wild-type mice. Consistent with increased hepatocyte Notch signaling in NASH, we observed a close correlation between markers of Notch activation and CCL2 expression in patients with NASH. We found that an evolutionarily conserved Notch/recombination signal binding protein for immunoglobulin kappa J region binding site in the Ccl2 promoter mediated transactivation of the Ccl2 promoter in NASH diet-fed mice. Increased liver MoMF infiltrate and liver fibrosis seen in opposite gain-of-function mice was ameliorated with concomitant hepatocyte Ccl2 knockout or CCR2 inhibitor treatment. Hepatocyte Notch activation prompts MCP-1-dependent increase in liver MoMF infiltration and fibrosis.
Keywords: Chemokines; Fibrosis; Gastroenterology; Metabolism.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

