Percutaneous MitraClip Device or Surgical Mitral Valve Repair in Patients With Primary Mitral Regurgitation Who Are Candidates for Surgery: Design and Rationale of the REPAIR MR Trial
- PMID: 36752231
- PMCID: PMC10111491
- DOI: 10.1161/JAHA.122.027504
Percutaneous MitraClip Device or Surgical Mitral Valve Repair in Patients With Primary Mitral Regurgitation Who Are Candidates for Surgery: Design and Rationale of the REPAIR MR Trial
Abstract
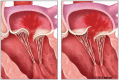
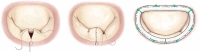
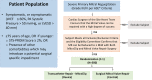
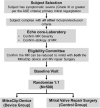
Background The current standard of care for the treatment of patients with primary mitral regurgitation (MR) is surgical mitral valve repair. Transcatheter edge-to-edge repair with the MitraClip device provides a less invasive treatment option for patients with both primary and secondary MR. Worldwide, >150 000 patients have been treated with the MitraClip device. However, in the United States, MitraClip is approved for use only in primary patients with MR who are at high or prohibitive risk for mitral valve surgery. The REPAIR MR (Percutaneous MitraClip Device or Surgical Mitral Valve Repair in Patients With Primary Mitral Regurgitation Who Are Candidates for Surgery) trial is designed to compare early and late outcomes associated with transcatheter edge-to-edge repair with the MitraClip and surgical repair of primary MR in older or moderate surgical risk patients. Methods and Results The REPAIR MR trial is a prospective, randomized, parallel-controlled, open-label multicenter, noninferiority trial for the treatment of severe primary MR (verified by an independent echocardiographic core laboratory). Patients with severe MR and indications for surgery because of symptoms (New York Heart Association class II-IV), or without symptoms with left ventricular ejection fraction ≤60%, pulmonary artery systolic pressure >50 mm Hg, or left ventricular end-systolic diameter ≥40 mm are eligible for the trial provided they meet the moderate surgical risk criteria as follows: (1) ≥75 years of age, or (2) if <75 years of age, then the subject has a Society of Thoracic Surgeons Predicted Risk Of Mortality score of ≥2% for mitral repair (or Society of Thoracic Surgeons replacement score of ≥4%), or the presence of a comorbidity that may introduce a surgery-specific risk. The local surgeon must determine that the mitral valve can be surgically repaired. Additionally, an independent eligibility committee will confirm that the MR can be reduced to mild or less with both the MitraClip and surgical mitral valve repair with a high degree of certainty. A total of 500 eligible subjects will be randomized in a 1:1 ratio to receive the MitraClip device or to undergo surgical mitral valve repair (control group). There are 2 co-primary end points for the trial, both of which will be evaluated at 2 years. Each subject will be followed for 10 years after enrollment. The study has received approval from both the Food and Drug Administration and the Centers for Medicare and Medicaid Services, and enrolled its first subject in July 2020. Conclusions The REPAIR MR trial will determine the safety and effectiveness of transcatheter edge-to-edge repair with the MitraClip in patients with primary MR who are at moderate surgical risk and are candidates for surgical MV repair. The trial will generate contemporary comparative clinical evidence for the MitraClip device and surgical MV repair. Registration https://clinicaltrials.gov/ct2/show/NCT04198870; NCT04198870.
Keywords: MitraClip; REPAIR MR; cardiovascular diseases; heart valve diseases; mitral regurgitation; mitral valve insufficiency; mitral valve repair.
Figures


References
-
- Mirabel M, Iung B, Baron G, Messika‐Zeitoun D, Detaint D, Vanoverschelde JL, Butchart EG, Ravaud P, Vahanian A. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J. 2007;28:1358–1365. doi: 10.1093/eurheartj/ehm001 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

