Chymase Inhibition Resolves and Prevents Deep Vein Thrombosis Without Increasing Bleeding Time in the Mouse Model
- PMID: 36752268
- PMCID: PMC10111474
- DOI: 10.1161/JAHA.122.028056
Chymase Inhibition Resolves and Prevents Deep Vein Thrombosis Without Increasing Bleeding Time in the Mouse Model
Abstract
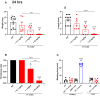
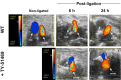
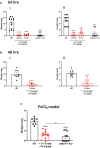
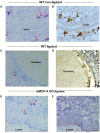
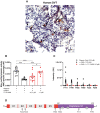
Background Deep vein thrombosis (DVT) is the primary cause of pulmonary embolism and the third most life-threatening cardiovascular disease in North America. Post-DVT anticoagulants, such as warfarin, heparin, and direct oral anticoagulants, reduce the incidence of subsequent venous thrombi. However, all currently used anticoagulants affect bleeding time at various degrees, and there is therefore a need for improved therapeutic regimens in DVT. It has recently been shown that mast cells play a crucial role in a DVT murine model. The underlying mechanism involved in the prothrombotic properties of mast cells, however, has yet to be identified. Methods and Results C57BL/6 mice and mouse mast cell protease-4 (mMCP-4) genetically depleted mice (mMCP-4 knockout) were used in 2 mouse models of DVT, partial ligation (stenosis) and ferric chloride-endothelial injury model of the inferior vena cava. Thrombus formation and impact of genetically repressed or pharmacologically (specific inhibitor TY-51469) inhibited mMCP-4 were evaluated by morphometric measurements of thrombi immunochemistry (mouse and human DVT), color Doppler ultrasound, bleeding times, and enzymatic activity assays ex vivo. Recombinant chymases, mMCP-4 (mouse) and CMA-1 (human), were used to characterize the interaction with murine and human plasmin, respectively, by mass spectrometry and enzymatic activity assays. Inhibiting mast cell-generated mMCP-4, genetically or pharmacologically, resolves and prevents venous thrombus formation in both DVT models. Inferior vena cava blood flow obstruction was observed in the stenosis model after 6 hours of ligation, in control- but not in TY-51469-treated mice. In addition, chymase inhibition had no impact on bleeding times of healthy or DVT mice. Furthermore, endogenous chymase limits plasmin activity in thrombi ex vivo. Recombinant mouse or human chymase degrades/inactivates purified plasmin in vitro. Finally, mast cell-containing immunoreactive chymase was identified in human DVT. Conclusions This study identified a major role for mMCP-4, a granule-localized protease of chymase type, in DVT formation. These findings support a novel pharmacological strategy to resolve or prevent DVT without affecting the coagulation cascade through the inhibition of chymase activity.
Keywords: TY‐51469; chymase; deep vein thrombosis; mouse; mouse mast cell protease‐4 knockout; plasmin.
Figures
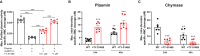
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

