A flexible carbon nanotube electrode array for acute in vivo EMG recordings
- PMID: 36752408
- PMCID: PMC10010927
- DOI: 10.1152/jn.00262.2022
A flexible carbon nanotube electrode array for acute in vivo EMG recordings
Abstract
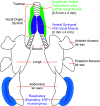
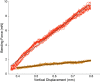
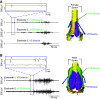
Executing complex behaviors requires precise control of muscle activity. Our understanding of how the nervous system learns and controls motor skills relies on recording electromyographic (EMG) signals from multiple muscles that are engaged in the motor task. Despite recent advances in tools for monitoring and manipulating neural activity, methods for recording in situ spiking activity in muscle fibers have changed little in recent decades. Here, we introduce a novel experimental approach to recording high-resolution EMG signals using parylene-coated carbon nanotube fibers (CNTFs). These fibers are fabricated via a wet spinning process and twisted together to create a bipolar electrode. Single CNTFs are strong, extremely flexible, small in diameter (14-24 µm), and have low interface impedance. We present two designs to build bipolar electrode arrays that, due to the small size of CNTF, lead to high spatial resolution EMG recordings. To test the EMG arrays, we recorded the activity of small (4 mm length) vocal muscles in songbirds in an acute setting. CNTF arrays were more flexible and yielded multiunit/bulk EMG recordings with higher SNR compared with stainless steel wire electrodes. Furthermore, we were able to record single-unit recordings not previously reported in these small muscles. CNTF electrodes are therefore well-suited for high-resolution EMG recording in acute settings, and we present both opportunities and challenges for their application in long-term chronic recordings.NEW & NOTEWORTHY We introduce a novel approach to record high-resolution EMG signals in small muscles using extremely strong and flexible carbon nanotube fibers (CNTFs). We test their functionality in songbird vocal muscles. Acute EMG recordings successfully yielded multiunit recordings with high SNR. Furthermore, they successfully isolated single-unit spike trains from CNTF recordings. CNTF electrodes have great potential for chronic EMG studies of small, deep muscles that demand high electrode flexibility and strength.
Keywords: carbon nanotube fibers; electromyography; motor systems; muscles; neurophysiology.
Conflict of interest statement
M.P. has a financial interest in DexMat, Inc., which is commercializing CNT fibers and threads. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures




Similar articles
-
Stable, long-term single-neuronal recording from the rat spinal cord with flexible carbon nanotube fiber electrodes.J Neural Eng. 2022 Sep 29;19(5). doi: 10.1088/1741-2552/ac9258. J Neural Eng. 2022. PMID: 36108593
-
Accurate and representative decoding of the neural drive to muscles in humans with multi-channel intramuscular thin-film electrodes.J Physiol. 2015 Sep 1;593(17):3789-804. doi: 10.1113/JP270902. J Physiol. 2015. PMID: 26174910 Free PMC article.
-
Pitfalls of intramuscular electromyographic recordings from the human costal diaphragm.Clin Neurophysiol. 2000 Aug;111(8):1420-4. doi: 10.1016/s1388-2457(00)00341-2. Clin Neurophysiol. 2000. PMID: 10904223
-
Recent progress in the diagnostic use of surface EMG for neurological diseases.J Electromyogr Kinesiol. 2000 Oct;10(5):287-91. doi: 10.1016/s1050-6411(00)00020-1. J Electromyogr Kinesiol. 2000. PMID: 11018438 Review.
-
State of the art review: Intravaginal probes for recording electromyography from the pelvic floor muscles.Neurourol Urodyn. 2015 Feb;34(2):104-12. doi: 10.1002/nau.22529. Epub 2013 Nov 21. Neurourol Urodyn. 2015. PMID: 24264797 Review.
Cited by
-
Myomatrix arrays for high-definition muscle recording.Elife. 2023 Dec 19;12:RP88551. doi: 10.7554/eLife.88551. Elife. 2023. PMID: 38113081 Free PMC article.
-
A synchrotron X-ray CT-based 3D atlas of the songbird syrinx with single muscle fibre resolution implies fine motor control of syringeal vocal folds.Philos Trans R Soc Lond B Biol Sci. 2025 Feb 27;380(1920):20230430. doi: 10.1098/rstb.2023.0430. Epub 2025 Feb 27. Philos Trans R Soc Lond B Biol Sci. 2025. PMID: 40010387
References
-
- Basmajian JV, Luca CJ. Muscles Alive: Their Functions Revealed by Electromyography. Baltimore, MD: Williams & Wilkins, 1985.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

