Test performance metrics for breast, cervical, colon, and lung cancer screening: a systematic review
- PMID: 36752508
- PMCID: PMC10086636
- DOI: 10.1093/jnci/djad028
Test performance metrics for breast, cervical, colon, and lung cancer screening: a systematic review
Abstract
Background: Multiple quality metrics have been recommended to ensure consistent, high-quality execution of screening tests for breast, cervical, colorectal, and lung cancers. However, minimal data exist evaluating the evidence base supporting these recommendations and the consistency of definitions and concepts included within and between cancer types.
Methods: We performed a systematic review for each cancer type using MEDLINE, Embase, and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) from 2010 to April 2020 to identify guidelines from screening programs or professional organizations containing quality metrics for tests used in breast, cervical, colorectal, and lung cancer screening. We abstracted metrics' definitions, target performance levels, and related supporting evidence for test completeness, adequacy (sufficient visualization or collection), accuracy, and safety.
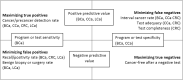
Results: We identified 11 relevant guidelines with 20 suggested quality metrics for breast cancer, 5 guidelines with 9 metrics for cervical cancer, 13 guidelines with 18 metrics for colorectal cancer (CRC), and 3 guidelines with 7 metrics for lung cancer. These included 54 metrics related to adequacy (n = 6), test completeness (n = 3), accuracy (n = 33), and safety (n = 12). Target performance levels were defined for 30 metrics (56%). Ten (19%) were supported by evidence, all from breast and CRC, with no evidence cited to support metrics from cervical and lung cancer screening.
Conclusions: Considerably more guideline-recommended test performance metrics exist for breast and CRC screening than cervical or lung cancer. The domains covered are inconsistent among cancers, and few targets are supported by evidence. Clearer evidence-based domains and targets are needed for test performance metrics.
Registration: PROSPERO 2020 CRD42020179139.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
Katharine Rendle reports grant funding from Pfizer and AstraZeneca (outside this work) and paid scientific advisory from Merck (outside this work). Debra Ritzwoller reports grant funding from Pfizer, outside of this work, paid to her institution. The other authors have no relevant conflicts of interest to disclose.
Figures

References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A.. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. - PubMed
-
- Lee B, Lin K, Liang PS.. Effectiveness and harms of colorectal cancer screening strategies. Gastrointest Endosc Clin N Am. 2022;32(2):215-226. - PubMed
-
- World Health Organization. Regional Office for Europe. Screening Programmes: A Short Guide. Increase Effectiveness, Maximize Benefits and Minimize Harm. Copenhagen: WHO Regional Office for Europe; 2020.
-
- Mainz J. Defining and classifying clinical indicators for quality improvement. Int J Qual Health Care. 2003;15(6):523-530. - PubMed
-
- Hermens RP, Ouwens MM, Vonk-Okhuijsen SY, et al.Development of quality indicators for diagnosis and treatment of patients with non-small cell lung cancer: a first step toward implementing a multidisciplinary, evidence-based guideline. Lung Cancer. 2006;54(1):117-124. - PubMed

