Cortisol/glucocorticoid receptor: a critical mediator of the ovulatory process and luteinization in human periovulatory follicles
- PMID: 36752644
- PMCID: PMC10068287
- DOI: 10.1093/humrep/dead017
Cortisol/glucocorticoid receptor: a critical mediator of the ovulatory process and luteinization in human periovulatory follicles
Abstract
Study question: Do cortisol/glucocorticoid receptors play an active role in the human ovary during ovulation and early luteinization?
Summary answer: The ovulatory hCG stimulation-induced glucocorticoid receptor signaling plays a crucial role in regulating steroidogenesis and ovulatory cascade in human periovulatory follicles.
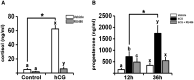
What is known already: Previous studies reported an increase in cortisol levels in the human follicular fluid after the LH surge or ovulatory hCG administration. However, little is known about the role of cortisol/glucocorticoid receptors in the ovulatory process and luteinization in humans.
Study design, size, duration: This study was an experimental prospective clinical and laboratory-based study. An in vivo experimental study was accomplished utilizing the dominant ovarian follicles from 38 premenopausal women undergoing laparoscopic sterilization. An in vitro experimental study was completed using the primary human granulosa/lutein cells (hGLC) from 26 premenopausal women undergoing IVF.
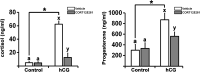
Participants/materials, setting, methods: This study was conducted in a private fertility clinic and academic medical centers. Dominant ovarian follicles were collected before the LH surge and at defined times after hCG administration from women undergoing laparoscopic sterilization. Primary hGLC were collected from women undergoing IVF. hGLC were treated without or with hCG in the absence or presence of RU486 (20 µM; dual antagonist for progesterone receptor and glucocorticoid receptor) or CORT125281 (50 µM; selective glucocorticoid receptor antagonist) for 12 or 36 h. The expression of genes involved in glucocorticoid receptor signaling, steroidogenesis, and ovulatory cascade was studied with RT-quantitative PCR and western blotting. The production of cortisol, corticosterone, and progesterone was assessed by hormone assay kits.
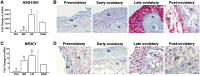
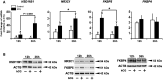
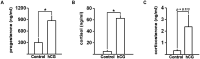
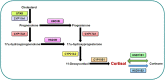
Main results and the role of chance: hCG administration upregulated the expression of hydroxysteroid 11-beta dehydrogenase 1 (HSD11B1), nuclear receptor subfamily 3 group C member 1 (NR3C1), FKBP prolyl isomerase 5 (FKBP5), and FKBP prolyl isomerase 4 (FKBP4) in human ovulatory follicles and in hGLC (P < 0.05). RU486 and CORT125281 reduced hCG-induced increases in progesterone and cortisol production in hGLC. The expression of genes involved in glucocorticoid receptor signaling, steroidogenesis, and the key ovulatory process was reduced by RU486 and/or CORT125281 in hGLC.
Large scale data: N/A.
Limitations, reasons for caution: The role of cortisol/glucocorticoid receptors demonstrated using the hGLC model may not fully reflect their physiological roles in vivo.
Wider implications of the findings: Successful ovulation and luteinization are essential for female fertility. Women with dysregulated cortisol levels often suffer from anovulatory infertility. Deciphering the functional role of glucocorticoid receptor signaling in human periovulatory follicles enhances our knowledge of basic ovarian physiology and may provide therapeutic insights into treating infertility in women.
Study funding/competing interest(s): This study was supported by P01HD71875 (to M.J., T.E.C., and M.B.) and R01HD096077 (to M.J.) from the Foundation for the National Institutes of Health and the BTPSRF of the University of Kentucky Markey Cancer Center (P30CA177558). The authors report no competing interests.
Trial registration number: N/A.
Keywords: cortisol; glucocorticoid receptor; granulosa cells; humans; luteinization; ovulation.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Akison LK, Robker RL.. The critical roles of progesterone receptor (PGR) in ovulation, oocyte developmental competence and oviductal transport in mammalian reproduction. Reprod Domest Anim 2012;47(Suppl 4):288–296. - PubMed
-
- Amin M, Simerman A, Cho M, Singh P, Briton-Jones C, Hill D, Grogan T, Elashoff D, Clarke NJ, Chazenbalk GD. et al. 21-Hydroxylase-derived steroids in follicles of nonobese women undergoing ovarian stimulation for in vitro fertilization (IVF) positively correlate with lipid content of luteinized granulosa cells (LGCs) as a source of cholesterol for steroid synthesis. J Clin Endocrinol Metab 2014;99:1299–1306. - PMC - PubMed
-
- Andersen CY, Morineau G, Fukuda M, Westergaard LG, Ingerslev HJ, Fiet J, Byskov AG.. Assessment of the follicular cortisol:cortisone ratio. Hum Reprod 1999;14:1563–1568. - PubMed
-
- Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology 2009;34(Suppl 1):S186–S195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

