An update on the long-term outcomes of prenatal dexamethasone treatment in congenital adrenal hyperplasia
- PMID: 36752813
- PMCID: PMC10083667
- DOI: 10.1530/EC-22-0400
An update on the long-term outcomes of prenatal dexamethasone treatment in congenital adrenal hyperplasia
Abstract
First-trimester prenatal treatment with glucocorticoid (GC) dexamethasone (DEX) in pregnancies at risk for classic congenital adrenal hyperplasia (CAH) is associated with ethical dilemmas. Though effective in reducing virilisation in girls with CAH, it entails exposure to high doses of GC in fetuses that do not benefit from the treatment. The current paper provides an update on the literature on outcomes of prenatal DEX treatment in CAH cases and unaffected subjects. Long-term follow-up research is still needed to determine treatment safety. In addition, advances in early prenatal diagnostics for CAH and sex-typing as well as studies assessing dosing effects of DEX may avoid unnecessary treatment and improve treatment safety.
Keywords: CAH; brain development; dexamethasone; first trimester; prenatal treatment; treatment safety.
Conflict of interest statement
The authors have nothing to disclose.
Figures
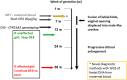

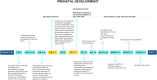
References
-
- Claahsen-van der Grinten HL, Speiser PW, Ahmed SF, Arlt W, Auchus RJ, Falhammar H, Flück CE, Guasti L, Huebner A, Kortmann BBMet al.Congenital adrenal hyperplasia-current insights in pathophysiology, diagnostics, and management. Endocrine Reviews 20224391–159. (10.1210/endrev/bnab016) - DOI - PMC - PubMed
-
- Speiser PW, Arlt W, Auchus RJ, Baskin LS, Conway GS, Merke DP, Meyer-Bahlburg HFL, Miller WL, Murad MH, Oberfield SEet al.Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 20181034043–4088. (10.1210/jc.2018-01865) - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

