Similar voltage-sensor movement in spHCN channels can cause closing, opening, or inactivation
- PMID: 36752823
- PMCID: PMC9948645
- DOI: 10.1085/jgp.202213170
Similar voltage-sensor movement in spHCN channels can cause closing, opening, or inactivation
Abstract
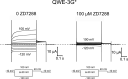
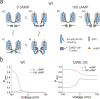
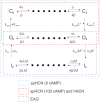
Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels contribute to the rhythmic firing of pacemaker neurons and cardiomyocytes. Mutations in HCN channels are associated with cardiac arrhythmia and epilepsy. HCN channels belong to the superfamily of voltage-gated K+ channels, most of which are activated by depolarization. HCN channels, however, are activated by hyperpolarization. The mechanism behind this reversed gating polarity of HCN channels is not clear. We here show that sea urchin HCN (spHCN) channels with mutations in the C-terminal part of the voltage sensor use the same voltage-sensor movement to either close or open in response to hyperpolarizations depending on the absence or presence of cAMP. Our results support that non-covalent interactions at the C-terminal end of the voltage sensor are critical for HCN gating polarity. These interactions are also critical for the proper closing of the channels because these mutations exhibit large constitutive currents. Since a similar voltage-sensor movement can cause both depolarization- and hyperpolarization-activation in the same channel, this suggests that the coupling between the voltage sensor and the pore is changed to create channels opened by different polarities. We also show an identical voltage-sensor movement in activated and inactivated spHCN channels and suggest a model for spHCN activation and inactivation. Our results suggest the possibility that channels open by opposite voltage dependence, such as HCN and the related EAG channels, use the same voltage-sensor movement but different coupling mechanisms between the voltage sensor and the gate.
© 2023 Wu et al.
Figures
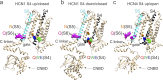






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

