IL-4 and helminth infection downregulate MINCLE-dependent macrophage response to mycobacteria and Th17 adjuvanticity
- PMID: 36753434
- PMCID: PMC9908076
- DOI: 10.7554/eLife.72923
IL-4 and helminth infection downregulate MINCLE-dependent macrophage response to mycobacteria and Th17 adjuvanticity
Abstract
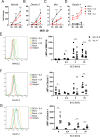
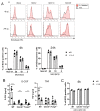
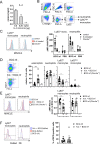
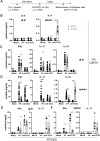
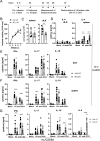
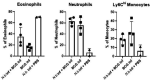
The myeloid C-type lectin receptor (CLR) MINCLE senses the mycobacterial cell wall component trehalose-6,6'-dimycolate (TDM). Recently, we found that IL-4 downregulates MINCLE expression in macrophages. IL-4 is a hallmark cytokine in helminth infections, which appear to increase the risk for mycobacterial infection and active tuberculosis. Here, we investigated functional consequences of IL-4 and helminth infection on MINCLE-driven macrophage activation and Th1/Th17 adjuvanticity. IL-4 inhibited MINCLE and cytokine induction after macrophage infection with Mycobacterium bovis bacille Calmette-Guerin (BCG). Infection of mice with BCG upregulated MINCLE on myeloid cells, which was inhibited by IL-4 plasmid injection and by infection with the nematode Nippostrongylus brasiliensis in monocytes. To determine the impact of helminth infection on MINCLE-dependent immune responses, we vaccinated mice with a recombinant protein together with the MINCLE ligand trehalose-6,6-dibehenate (TDB) as adjuvant. Concurrent infection with N. brasiliensis or with Schistosoma mansoni promoted T cell-derived IL-4 production and suppressed Th1/Th17 differentiation in the spleen. In contrast, helminth infection did not reduce Th1/Th17 induction by TDB in draining peripheral lymph nodes, where IL-4 levels were unaltered. Upon use of the TLR4-dependent adjuvant G3D6A, N. brasiliensis infection impaired selectively the induction of splenic antigen-specific Th1 but not of Th17 cells. Inhibition of MINCLE-dependent Th1/Th17 responses in mice infected with N. brasiliensis was dependent on IL-4/IL-13. Thus, helminth infection attenuated the Th17 response to MINCLE-dependent immunization in an organ- and adjuvant-specific manner via the Th2 cytokines IL-4/IL-13. Taken together, our results demonstrate downregulation of MINCLE expression on monocytes and macrophages by IL-4 as a possible mechanism of thwarted Th17 vaccination responses by underlying helminth infection.
Keywords: C-type lectin receptors; Nippostrongylus brasiliensis; Schistosoma mansoni; Th cell differentiation; co-infection; immunology; infectious disease; inflammation; microbiology; mouse; mycobacterial cord factor.
© 2023, Schick et al.
Conflict of interest statement
JS, MA, ML, NM, SW, JS, CS, BB, DC, CA, SW, DV, Cd, RL No competing interests declared
Figures


References
-
- Abate E, Belayneh M, Idh J, Diro E, Elias D, Britton S, Aseffa A, Stendahl O, Schön T. Asymptomatic helminth infection in active tuberculosis is associated with increased regulatory and Th-2 responses and a lower sputum smear positivity. PLOS Neglected Tropical Diseases. 2015;9:e0003994. doi: 10.1371/journal.pntd.0003994. - DOI - PMC - PubMed
-
- Agger EM, Rosenkrands I, Hansen J, Brahimi K, Vandahl BS, Aagaard C, Werninghaus K, Kirschning C, Lang R, Christensen D, Theisen M, Follmann F, Andersen P. Cationic liposomes formulated with synthetic mycobacterial cordfactor (CAF01): a versatile adjuvant for vaccines with different immunological requirements. PLOS ONE. 2008;3:e3116. doi: 10.1371/journal.pone.0003116. - DOI - PMC - PubMed
-
- Babu S, Bhat SQ, Kumar NP, Anuradha R, Kumaran P, Gopi PG, Kolappan C, Kumaraswami V, Nutman TB. Attenuation of toll-like receptor expression and function in latent tuberculosis by coexistent filarial infection with restoration following antifilarial chemotherapy. PLOS Neglected Tropical Diseases. 2009a;3:e489. doi: 10.1371/journal.pntd.0000489. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

