Development and evaluation of a multiplex serodiagnostic bead assay (BurkPx) for accurate melioidosis diagnosis
- PMID: 36753506
- PMCID: PMC9907819
- DOI: 10.1371/journal.pntd.0011072
Development and evaluation of a multiplex serodiagnostic bead assay (BurkPx) for accurate melioidosis diagnosis
Abstract
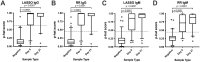
Burkholderia pseudomallei, the causative agent of melioidosis, is a gram-negative soil bacterium well recognized in Southeast Asia and northern Australia. However, wider and expanding global distribution of B. pseudomallei has been elucidated. Early diagnosis is critical for commencing the specific therapy required to optimize outcome. Serological testing using the indirect hemagglutination (IHA) antibody assay has long been used to augment diagnosis of melioidosis and to monitor progress. However, cross reactivity and prior exposure may complicate the diagnosis of current clinical disease (melioidosis). The goal of our study was to develop and initially evaluate a serology assay (BurkPx) that capitalized upon host response to multiple antigens. Antigens were selected from previous studies for expression/purification and conjugation to microspheres for multiantigen analysis. Selected serum samples from non-melioidosis controls and serial samples from culture-confirmed melioidosis patients were used to characterize the diagnostic power of individual and combined antigens at two times post admission. Multiple variable models were developed to evaluate multivariate antigen reactivity, identify important antigens, and determine sensitivity and specificity for the diagnosis of melioidosis. The final multiplex assay had a diagnostic sensitivity of 90% and specificity of 93%, which was superior to any single antigen in side-by-side comparisons. The sensitivity of the assay started at >85% for the initial serum sample after admission and increased to 94% 21 days later. Weighting antigen contribution to each model indicated that certain antigen contributed to diagnosis more than others, which suggests that the number of antigens in the assay can be decreased. In summation, the BurkPx assay can facilitate the diagnosis of melioidosis and potentially improve on currently available serology assays. Further evaluation is now required in both melioidosis-endemic and non-endemic settings.
Copyright: © 2023 Settles et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

