Metabolite asymmetric dimethylarginine (ADMA) functions as a destabilization enhancer of SOX9 mediated by DDAH1 in osteoarthritis
- PMID: 36753544
- PMCID: PMC9908022
- DOI: 10.1126/sciadv.ade5584
Metabolite asymmetric dimethylarginine (ADMA) functions as a destabilization enhancer of SOX9 mediated by DDAH1 in osteoarthritis
Abstract
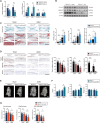
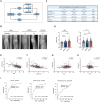
Osteoarthritis (OA) is a degenerative disease with a series of metabolic changes accompanied by many altered enzymes. Here, we report that the down-regulated dimethylarginine dimethylaminohydrolase-1 (DDAH1) is accompanied by increased asymmetric dimethylarginine (ADMA) in degenerated chondrocytes and in OA samples. Global or chondrocyte-conditional knockout of ADMA hydrolase DDAH1 accelerated OA development in mice. ADMA induces the degeneration and senescence of chondrocytes and reduces the extracellular matrix deposition, thereby accelerating OA progression. ADMA simultaneously binds to SOX9 and its deubiquitinating enzyme USP7, blocking the deubiquitination effects of USP7 on SOX9 and therefore leads to SOX9 degradation. The ADMA level in synovial fluids of patients with OA is increased and has predictive value for OA diagnosis with good sensitivity and specificity. Therefore, activating DDAH1 to reduce ADMA level might be a potential therapeutic strategy for OA treatment.
Figures



References
-
- M. Kapoor, J. Martel-Pelletier, D. Lajeunesse, J. P. Pelletier, H. Fahmi, Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 7, 33–42 (2011). - PubMed
-
- S. Farnaghi, I. Prasadam, G. Cai, T. Friis, Z. Du, R. Crawford, X. Mao, Y. Xiao, Protective effects of mitochondria-targeted antioxidants and statins on cholesterol-induced osteoarthritis. FASEB J. 31, 356–367 (2017). - PubMed
-
- W. S. Choi, G. Lee, W. H. Song, J. T. Koh, J. Yang, J. S. Kwak, H. E. Kim, S. K. Kim, Y. O. Son, H. Nam, I. Jin, Z. Y. Park, J. Kim, I. Y. Park, J. I. Hong, H. A. Kim, C. H. Chun, J. H. Ryu, J. S. Chun, The CH25H-CYP7B1-RORα axis of cholesterol metabolism regulates osteoarthritis. Nature 566, 254–258 (2019). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

