Spinal cord tissue engineering via covalent interaction between biomaterials and cells
- PMID: 36753555
- PMCID: PMC9908024
- DOI: 10.1126/sciadv.ade8829
Spinal cord tissue engineering via covalent interaction between biomaterials and cells
Abstract
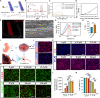
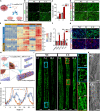
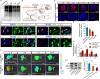
Noncovalent interactions between cells and environmental cues have been recognized as fundamental physiological interactions that regulate cell behavior. However, the effects of the covalent interactions between cells and biomaterials on cell behavior have not been examined. Here, we demonstrate a combined strategy based on covalent conjugation between biomaterials (collagen fibers/lipid nanoparticles) and various cells (exogenous neural progenitor cells/astrocytes/endogenous tissue-resident cells) to promote neural regeneration after spinal cord injury (SCI). We found that metabolic azido-labeled human neural progenitor cells conjugated on dibenzocyclooctyne-modified collagen fibers significantly promoted cell adhesion, spreading, and differentiation compared with noncovalent adhesion. In addition, dibenzocyclooctyne-modified lipid nanoparticles containing edaravone, a well-known ROS scavenger, could target azide-labeled spinal cord tissues or transplanted azide-modified astrocytes to improve the SCI microenvironment. The combined application of these covalent conjugation strategies in a rat SCI model boosted neural regeneration, suggesting that the covalent interactions between cells and biomaterials have great potential for tissue regeneration.
Figures





References
-
- Z. Alvarez, A. N. Kolberg-Edelbrock, I. R. Sasselli, J. A. Ortega, R. Qiu, Z. Syrgiannis, P. A. Mirau, F. Chen, S. M. Chin, S. Weigand, E. Kiskinis, S. I. Stupp, Bioactive scaffolds with enhanced supramolecular motion promote recovery from spinal cord injury. Science 374, 848–856 (2021). - PMC - PubMed
-
- X. L. Wang, N. Rivera-Bolanos, B. Jiang, G. A. Ameer, Advanced functional biomaterials for stem cell delivery in regenerative engineering and medicine. Adv. Funct. Mater. 29, 1809009 (2019).
-
- A. P. Liu, E. A. Appel, P. D. Ashby, B. M. Baker, E. Franco, L. Gu, K. Haynes, N. S. Joshi, A. M. Kloxin, P. H. J. Kouwer, J. Mittal, L. Morsut, V. Noireaux, S. Parekh, R. Schulman, S. K. Y. Tang, M. T. Valentine, S. L. Vega, W. Weber, N. Stephanopoulos, O. Chaudhuri, The living interface between synthetic biology and biomaterial design. Nat. Mater. 21, 390–397 (2022). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

