Cortical microtubules contribute to division plane positioning during telophase in maize
- PMID: 36753568
- PMCID: PMC10118269
- DOI: 10.1093/plcell/koad033
Cortical microtubules contribute to division plane positioning during telophase in maize
Abstract
Cell divisions are accurately positioned to generate cells of the correct size and shape. In plant cells, the new cell wall is built in the middle of the cell by vesicles trafficked along an antiparallel microtubule and a microfilament array called the phragmoplast. The phragmoplast expands toward a specific location at the cell cortex called the division site, but how it accurately reaches the division site is unclear. We observed microtubule arrays that accumulate at the cell cortex during the telophase transition in maize (Zea mays) leaf epidermal cells. Before the phragmoplast reaches the cell cortex, these cortical-telophase microtubules transiently interact with the division site. Increased microtubule plus end capture and pausing occur when microtubules contact the division site-localized protein TANGLED1 or other closely associated proteins. Microtubule capture and pausing align the cortical microtubules perpendicular to the division site during telophase. Once the phragmoplast reaches the cell cortex, cortical-telophase microtubules are incorporated into the phragmoplast primarily by parallel bundling. The addition of microtubules into the phragmoplast promotes fine-tuning of the positioning at the division site. Our hypothesis is that division site-localized proteins such as TANGLED1 organize cortical microtubules during telophase to mediate phragmoplast positioning at the final division plane.
© The Author(s) 2023. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures




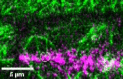




Comment in
-
Against the grain: Certain microtubules arrange perpendicularly to the division site to guide cell division in maize.Plant Cell. 2023 Apr 20;35(5):1302-1303. doi: 10.1093/plcell/koad039. Plant Cell. 2023. PMID: 36788714 Free PMC article. No abstract available.
References
-
- Buschmann H, Dols J, Kopischke S, Peña EJ, Andrade-Navarro MA, Heinlein M, Szymanski DB, Zachgo S, Doonan JH, Lloyd CW. Arabidopsis KCBP interacts with AIR9 but stays in the cortical division zone throughout mitosis via its MyTH4-FERM domain. J Cell Sci. 2015:128(11):2033–2046. 10.1242/jcs.156570 - DOI - PubMed

