Splenectomy improves erythrocyte functionality in spherocytosis based on septin abundance, but not maturation defects
- PMID: 36753606
- PMCID: PMC10468371
- DOI: 10.1182/bloodadvances.2022009114
Splenectomy improves erythrocyte functionality in spherocytosis based on septin abundance, but not maturation defects
Abstract
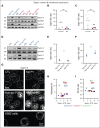
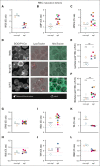
Splenectomy improves the clinical parameters of patients with hereditary spherocytosis, but its potential benefit to red blood cell (RBC) functionality and the mechanism behind this benefit remain largely overlooked. Here, we compared 7 nonsplenectomized and 13 splenectomized patients with mutations in the β-spectrin or the ankyrin gene. We showed that hematological parameters, spherocyte abundance, osmotic fragility, intracellular calcium, and extracellular vesicle release were largely but not completely restored by splenectomy, whereas cryohemolysis was not. Affected RBCs exhibited decreases in β-spectrin and/or ankyrin contents and slight alterations in spectrin membrane distribution, depending on the mutation. These modifications were found in both splenectomized and nonsplenectomized patients and poorly correlated with RBC functionality alteration, suggesting additional impairments. Accordingly, we found an increased abundance of septins, small guanosine triphosphate-binding cytoskeletal proteins. Septins-2, -7, and -8 but not -11 were less abundant upon splenectomy and correlated with the disease severity. Septin-2 membrane association was confirmed by immunolabeling. Except for cryohemolysis, all parameters of RBC morphology and functionality correlated with septin abundance. The increased septin content might result from RBC maturation defects, as evidenced by (1) the decreased protein 4.2 and Rh-associated glycoprotein content in all patient RBCs, (2) increased endoplasmic reticulum remnants and endocytosis proteins in nonsplenectomized patients, and (3) increased lysosomal and mitochondrial remnants in splenectomized patients. Our study paves the way for a better understanding of the involvement of septins in RBC membrane biophysical properties. In addition, the lack of restoration of septin-independent cryohemolysis by splenectomy may call into question its recommendation in specific cases.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures





References
-
- Baines AJ. The spectrin-ankyrin-4.1-adducin membrane skeleton: adapting eukaryotic cells to the demands of animal life. Protoplasma. 2010;244(1-4):99–131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

