Brexucabtagene Autoleucel for Relapsed or Refractory Mantle Cell Lymphoma in Standard-of-Care Practice: Results From the US Lymphoma CAR T Consortium
- PMID: 36753699
- PMCID: PMC10489553
- DOI: 10.1200/JCO.22.01797
Brexucabtagene Autoleucel for Relapsed or Refractory Mantle Cell Lymphoma in Standard-of-Care Practice: Results From the US Lymphoma CAR T Consortium
Abstract
Purpose: Brexucabtagene autoleucel (brexu-cel) is an autologous CD19-directed chimeric antigen receptor (CAR) T-cell therapy approved for relapsed/refractory mantle cell lymphoma (MCL). This therapy was approved on the basis of the single-arm phase II ZUMA-2 trial, which showed best overall and complete response rates of 91% and 68%, respectively. We report clinical outcomes with brexu-cel in the standard-of-care setting for the approved indication.
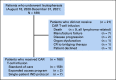
Patients and methods: Patients who underwent leukapheresis between August 1, 2020 and December 31, 2021, at 16 US institutions, with an intent to manufacture commercial brexu-cel for relapsed/refractory MCL, were included. Patient data were collected for analyses of responses, outcomes, and toxicities as per standard guidelines.
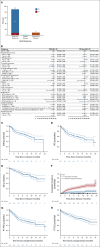
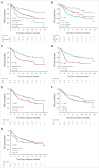
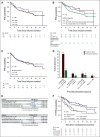
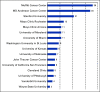
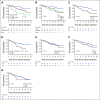
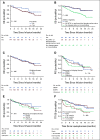
Results: Of 189 patients who underwent leukapheresis, 168 (89%) received brexu-cel infusion. Of leukapheresed patients, 79% would not have met ZUMA-2 eligibility criteria. Best overall and complete response rates were 90% and 82%, respectively. At a median follow-up of 14.3 months after infusion, the estimates for 6- and 12-month progression-free survival (PFS) were 69% (95% CI, 61 to 75) and 59% (95% CI, 51 to 66), respectively. The nonrelapse mortality was 9.1% at 1 year, primarily because of infections. Grade 3 or higher cytokine release syndrome and neurotoxicity occurred in 8% and 32%, respectively. In univariable analysis, high-risk simplified MCL international prognostic index, high Ki-67, TP53 aberration, complex karyotype, and blastoid/pleomorphic variant were associated with shorter PFS after brexu-cel infusion. Patients with recent bendamustine exposure (within 24 months before leukapheresis) had shorter PFS and overall survival after leukapheresis in intention-to-treat univariable analysis.
Conclusion: In the standard-of-care setting, the efficacy and toxicity of brexu-cel were consistent with those reported in the ZUMA-2 trial. Tumor-intrinsic features of MCL, and possibly recent bendamustine exposure, may be associated with inferior efficacy outcomes.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
References
-
- Jain P, Wang ML: Mantle cell lymphoma in 2022-A comprehensive update on molecular pathogenesis, risk stratification, clinical approach, and current and novel treatments. Am J Hematol 97:638-656, 2022 - PubMed
-
- Jain P, Dreyling M, Seymour JF, et al. : High-risk mantle cell lymphoma: Definition, current challenges, and management. J Clin Oncol 38:4302-4316, 2020 - PubMed
-
- Hoster E, Dreyling M, Klapper W, et al. : A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood 111:558-565, 2008 - PubMed
-
- Hoster E, Klapper W, Hermine O, et al. : Confirmation of the mantle-cell lymphoma International Prognostic Index in randomized trials of the European Mantle-Cell Lymphoma Network. J Clin Oncol 32:1338-1346, 2014 - PubMed
-
- Hoster E, Rosenwald A, Berger F, et al. : Prognostic value of Ki-67 index, cytology, and growth pattern in mantle-cell lymphoma: Results from randomized trials of the European Mantle Cell Lymphoma Network. J Clin Oncol 34:1386-1394, 2016 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

