Cancer burden in adolescents and young adults in Europe
- PMID: 36753992
- PMCID: PMC10024081
- DOI: 10.1016/j.esmoop.2022.100744
Cancer burden in adolescents and young adults in Europe
Abstract
Background: Cancer epidemiology is unique in adolescents and young adults (AYAs; aged 15-39 years). The European Society for Medical Oncology/European Society for Paediatric Oncology (ESMO/SIOPE) AYA Working Group aims to describe the burden of cancers in AYAs in Europe and across European Union (EU) countries.
Patients and methods: We used data available on the Global Cancer Observatory. We retrieved crude and age-standardised (World Standard Population) incidence and mortality rates. We reported about AYA cancer burden in Europe and between 28 EU member states. We described incidence and mortality for all cancers and for the 13 cancers most relevant to the AYA population.
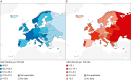
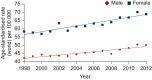
Results: Incidence and mortality varied widely between countries with the highest mortality observed in Eastern EU countries. Cancers of the female breast, thyroid and male testis were the most common cancers across countries followed by melanoma of skin and cancers of the cervix. Variations in cancer incidence rates across different populations may reflect different distribution of risk factors, variations in the implementation or uptake of screening as well as overdiagnosis. AYA cancer mortality disparities may be due to variation in early-stage diagnoses, different public education and awareness of cancer symptoms, different degrees of access or availability of treatment.
Conclusions: Our results highlight the future health care needs and requirements for AYA-specialised services to ensure a homogeneous treatment across different countries as well as the urgency for preventive initiatives that can mitigate the increasing burden.
Keywords: adolescents and young adults; cancer; incidence; mortality; population-based cancer registry.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure DS reports receipt of honoraria for providing academic peer review for research programme from French INCa, non-financial interest for advisory role in the clinical reference group advising NHS England about cancer policy in children and young people and non-remunerated membership of the Association of Cancer Physicians of the UK, SIOP Europe Adolescent Cancer Committee; IBS reports receipt of honoraria for participation in Advisory Board from Roche, receipt of honoraria as invited speaker from AstraZeneca, Novartis, Pfizer, Roche, receipt of personal and institutional financial interest as local PI from Novartis, Roche, non-financial interest for serving as local PI in EORTC Breast Group, OncoDistinct studies and non-remunerated advisory role in the Ethical Committee Serbia, National Agency for Drug Registration Serbia, the Working Group for Oncology of the Ministry of Health Serbia; NG reports receipt of honoraria to institution for participation in Advisory Board from Y-mAbs Therapeutics, receipt of financial support to institution from Eisai for covering travel expenses and registration fees as invited speaker at international meeting for the presentation of the results from the studies as international PI, receipt of consultancy fee to institution from Ipsen, non-remunerated Co-chair of the Fostering Age inclusive Research (FAIR) trial group of ACCELERATE; non-remunerated member of the Executive Committee of EEC (Euro-Wing Consortium), non-financial interest for leadership role as Chair of the FOSTER Consortium (Fight Osteosarcoma Through European Research) and non-remunerated member of GO-AJA, SFCE, SIOP Europe; FP reports receipt of honoraria for participation in Advisory Board from Roche Diagnostics, receipt of honoraria for providing expert testimony from Ipsen, Merck and non-financial interest for leadership role as Scientific Director of the European School of Oncology; ATo reports receipt of honoraria for participation in Advisory Board from MSD, AstraZeneca, Pfizer, Eli Lilly, receipt of honoraria as invited speaker from Eli Lilly, Novartis and non-remunerated member of AIOM; KS reports receipt of honoraria for participation in Advisory Board from Bayer, Novartis, Novo Nordisk, Roche, non-financial interest for leadership role in PanCare, SPOG, SSPHO and non-remunerated member of Board of Directors of SIOP Europe; GM reports receipt of honoraria for participation in Advisory Board from BMS, Janssen, Roche, Takeda, and receipt of honoraria as invited speaker from Amgen, AstraZeneca, MSD, Novartis, Pfizer; SB reports receipt of honoraria for participation in Advisory Board from Bayer Healthcare, Boehringer Ingelheim, Eli Lilly, Hofmann La Roche, MAP-Biopharma; non-financial interest as principal investigator of the Bayer’s larotrectinib study; and non-remunerated member of the European Musculoskeletal Oncology Society (EMSOS), German Pediatric Oncology Society (GPOH); ES reports receipt of honoraria for participation in Advisory Board from AstraZeneca A.E., AstraZeneca UK Ltd., Gilead Sciences Hellas, Merck, Sharp & Dohme, Pfizer Hellas, receipt of honoraria as invited speaker from Amgen Hellas, Pfizer Hellas, receipt of honoraria for providing expert testimony from Ipsen and non-remunerated member of Board of Directors of Hellenic Oncology Research Group, Hellenic Society of Medical Oncology. All other authors have declared no conflicts of interest.
Figures
References
-
- What should the age range be for AYA oncology? J Adolesc Young Adult Oncol. 2011;1(1):3–10. - PubMed
-
- Fern L.A., Lewandowski J.A., Coxon K.M., Whelan J., National Cancer Research Institute Teenage and Young Adult Clinical Studies Group, UK Available, accessible, aware, appropriate, and acceptable: a strategy to improve participation of teenagers and young adults in cancer trials. Lancet Oncol. 2014;15(8):e341–e350. - PubMed
-
- Abrams A.N., Hazen E.P., Penson R.T. Psychosocial issues in adolescents with cancer. Cancer Treat Rev. 2007;33(7):622–630. - PubMed

