Local type 2 immunity in eosinophilic gastritis
- PMID: 36754294
- PMCID: PMC10330288
- DOI: 10.1016/j.jaci.2023.01.021
Local type 2 immunity in eosinophilic gastritis
Abstract
Background: Eosinophilic gastritis (EoG) associates with type 2 immunity. However, the type 2 cytokine cellular source, gastric T-cell composition, and gastric T-cell relationship (or relationships) with disease pathology remain understudied.
Objective: We defined gastric T-cell populations and their association with histologic and endoscopic EoG pathology.
Methods: Gastric biopsy samples (n = 6 EoG, n = 7 control) were subjected to histologic, endoscopic, and flow cytometry analyses. In a complementary cohort (n = 83 EoG), IL4, IL5, and IL13 mRNA levels were correlated with EoG pathologic parameters.
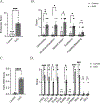
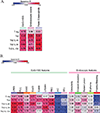
Results: Gastric biopsy samples contained CD3+ T cells that were mainly CD8+; the CD8/CD4 ratio was comparable in EoG and control biopsy samples (5.7 ± 3.0 and 4.3 ± 0.6, respectively; P = .28). Gastric regulatory T (CD3+CD4+FOXP3+) and TH2 (CD3+CD4+GATA3+) cell levels were increased in EoG versus controls (2-fold, P < .05 and 10-fold, P < .001, respectively) and correlated with gastric eosinophil levels (r = 0.63, P < .05 and r = 0.85, P < .001, respectively), endoscopic pathology (r = 0.56, P < .01; r = 0.84, P < .001, respectively), and histopathology (r = 0.72, P < .01; r = 0.82, P < .01, respectively). Cytokine-positive, most notably IL-4+, TH2 cell levels strongly correlated with histologic and endoscopic scores (r = 0.82, P < .0001 and r = 0.78, P < .0001, respectively). In an independent EoG cohort (n = 83), bulk gastric IL4, IL5, and IL13 mRNA levels correlated with histologic score (r = 0.22, P < .005; r = 0.54, P < .0001; and r = 0.36, P < .0001, respectively) and endoscopic score (r = 0.27, P < .001; r = 0.40, P < .0001; and r = 0.35, P < .0001, respectively).
Conclusions: EoG is a TH2 cell-associated disease featuring increased gastric type 2 cytokine-producing CD3+CD4+GATA3+TH2 cells that strongly correlate with disease pathologies.
Keywords: Eosinophilic gastritis; T cells; eosinophils; type 2 cytokines.
Copyright © 2023 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Competing interests:
M.E.R. is a consultant for Pulm One, Spoon Guru, ClostraBio, Serpin Pharm, Allakos, Celldex, Nextstone One, Bristol Myers Squibb, Astra Zeneca, Ellodi Pharma, GlaxoSmith Kline, Regeneron/Sanofi, Revolo Biotherapeutics, and Guidepoint and has an equity interest in the first seven listed and royalties from reslizumab (Teva Pharmaceuticals), PEESSv2 (Mapi Research Trust) and UpToDate. M.E.R. is an inventor of patents owned by Cincinnati Children’s Hospital Medical Center. T.S. has received research support from NIH (K99 AI158660) Research Fellowships and is a co-inventor of patents owned by Cincinnati Children’s Hospital Medical Center. M.H.C. has received research funding from AstraZeneca, Meritage Pharma Inc., Receptos/Celgene, Regeneron Pharmaceuticals and Shire, a Takeda company, and is a consultant for Allakos, Arena Pharmaceuticals, AstraZeneca, Calypso Biotech, EsoCap Biotech, GlaxoSmithKline, Receptos/Celgene/BMS, Regeneron Pharmaceuticals, Robarts Clinical Trials Inc./Alimentiv, Inc. and Shire, a Takeda company. V.A.M. is a consultant for Takeda and Allakos. All other authors declare that they have no competing interests.
Figures




References
-
- Moawad FJ. Eosinophilic Esophagitis: Incidence and Prevalence. Gastrointest Endosc Clin N Am 2018; 28:15–25. - PubMed
-
- Gonsalves N Eosinophilic Gastrointestinal Disorders. Clin Rev Allergy Immunol 2019; 57:272–85. - PubMed
-
- Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol 2004; 113:11–28; quiz 9. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

