Gallic acid diminishes pro-inflammatory interferon-γ- and interleukin-17-producing sub-populations in vitro in patients with psoriasis
- PMID: 36754913
- PMCID: PMC10185625
- DOI: 10.1007/s12026-023-09361-9
Gallic acid diminishes pro-inflammatory interferon-γ- and interleukin-17-producing sub-populations in vitro in patients with psoriasis
Abstract
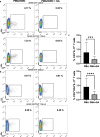
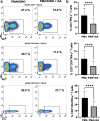
Psoriasis is an inflammation of the skin mediated via the IL-23/Thl17/IL-17 pathway. We have previously demonstrated that the anthocyanin delphinidin diminishes in vitro the IL-17 and IFN-γ production of peripheral monocytes isolated by psoriasis patients (PBMCs). The degradation product of delphinidin is gallic acid (GA). This phenolic acid compound found in fruits, red wine, or green tea exerts pleiotropic antioxidant, anticarcinogenic, antimicrobial, and anti-inflammatory properties. Previous research has demonstrated the inhibitory effect of GA on pro-inflammatory transcription factors, such as STAT3, RORγt, and NF-κB, or cytokines as IL-1β and TNF, which contribute to psoriasis development. We investigated the effect of GA in vitro on PBMCs, which were stimulated ex vivo, from 40 individuals (28 diagnosed with psoriasis vulgaris and 12 healthy controls (HCs)). In our experiments, PBMCs were cultured untreated or were activated in the presence of phorbol 12-myristate 13-acetate/ionomycin with or without GA. We utilized multicolor flow cytometry to assess the production of inteleukin-17 (IL-17) and interferon-γ (IFN-γ) in T and NK cells. GA did not alter the fractions of IL-17- or IFN-γ-producing T and IFN-γ-producing NK cells in HCs. However, in psoriasis patients, the effect of GA on that cell population was significant. Specifically, GA decreased the frequency of IL-17-producing cells within the CD3+ (T) and CD3+CD4+ (Th) compartment; the frequency of IFN-γ-producing cells within the CD3+, CD3+CD4+, and CD3+CD4- (Tc) compartment, and the frequency of IFN-γ-producing cells within the CD3-CD56+ (NK) compartment. Whether GA's effect also appears in vivo needs to be investigated in future.
Keywords: Flow cytometry; NK; NKT; PBMCs; Th1; Th17.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Delphinidin diminishes in vitro interferon-γ and interleukin-17 producing cells in patients with psoriatic disease.Immunol Res. 2022 Apr;70(2):161-173. doi: 10.1007/s12026-021-09251-y. Epub 2021 Nov 25. Immunol Res. 2022. PMID: 34825313
-
Decreased frequency of interferon-gamma- and interleukin-2-producing cells in patients with atopic diseases measured at the single cell level.J Allergy Clin Immunol. 1995 Oct;96(4):515-27. doi: 10.1016/s0091-6749(95)70296-2. J Allergy Clin Immunol. 1995. PMID: 7560664
-
Cannabidiol Mediates In Vitro Attenuation of Proinflammatory Cytokine Responses in Psoriatic Disease.Cannabis Cannabinoid Res. 2024 Feb;9(1):134-146. doi: 10.1089/can.2023.0237. Epub 2024 Jan 5. Cannabis Cannabinoid Res. 2024. PMID: 38181167
-
Increased in vitro induced CD4+ and CD8+ T cell IFN-gamma and CD4+ T cell IL-10 production in stable relapsing multiple sclerosis.Int J Neurosci. 1997 Aug;90(3-4):187-202. doi: 10.3109/00207459709000638. Int J Neurosci. 1997. PMID: 9352427 Review.
-
From IL-17 to IFN-γ in inflammatory skin disorders: Is transdifferentiation a potential treatment target?Front Immunol. 2022 Jul 28;13:932265. doi: 10.3389/fimmu.2022.932265. eCollection 2022. Front Immunol. 2022. PMID: 35967358 Free PMC article. Review.
Cited by
-
Protective Effects of Gallic Acid Against Lead Acetate- Induced Toxicity in Mice Ovary: Focus on Apoptosis, Inflammation, and Folliculogenesis.Food Sci Nutr. 2025 Jul 16;13(7):e70638. doi: 10.1002/fsn3.70638. eCollection 2025 Jul. Food Sci Nutr. 2025. PMID: 40678332 Free PMC article.
-
Emerging insights into the role of natural products and miRNAs in psoriasis: from pathophysiology to precision medicine.Naunyn Schmiedebergs Arch Pharmacol. 2025 Mar;398(3):2487-2509. doi: 10.1007/s00210-024-03528-3. Epub 2024 Oct 28. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39466441 Review.
-
Exploring the Therapeutic Potential of Natural Compounds in Psoriasis and Their Inclusion in Nanotechnological Systems.Antioxidants (Basel). 2024 Jul 28;13(8):912. doi: 10.3390/antiox13080912. Antioxidants (Basel). 2024. PMID: 39199158 Free PMC article. Review.
-
Flavonoids as Natural Anti-Inflammatory Agents in the Atopic Dermatitis Treatment.Pharmaceutics. 2025 Feb 15;17(2):261. doi: 10.3390/pharmaceutics17020261. Pharmaceutics. 2025. PMID: 40006628 Free PMC article. Review.
-
In vitro study of the treatment of dentin hypersensitivity with gallic acid combined with sodium fluoride.BMC Oral Health. 2024 Oct 30;24(1):1319. doi: 10.1186/s12903-024-05098-5. BMC Oral Health. 2024. PMID: 39478528 Free PMC article.
References
-
- Chiricozzi A, Romanelli P, Volpe E, Borsellino G, Romanelli M. Scanning the immunopathogenesis of psoriasis [Internet]. Int. J. Mol. Sci. MDPI AG; 2018 [cited 2021 Mar 31]. Available from: https://pubmed.ncbi.nlm.nih.gov/29316717/. - PMC - PubMed
-
- Goldminz AM, Au SC, Kim N, Gottlieb AB, Lizzul PF. NF-κB: an essential transcription factor in psoriasis. J Dermatol Sci. 2013;69:89–94. - PubMed
-
- Kamali AN, Noorbakhsh SM, Hamedifar H, Jadidi-Niaragh F, Yazdani R, Bautista JM, et al. A role for Th1-like Th17 cells in the pathogenesis of inflammatory and autoimmune disorders. Mol Immunol England. 2019;105:107–115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

