Key recommendations for primary care from the 2022 Global Initiative for Asthma (GINA) update
- PMID: 36754956
- PMCID: PMC9907191
- DOI: 10.1038/s41533-023-00330-1
Key recommendations for primary care from the 2022 Global Initiative for Asthma (GINA) update
Abstract
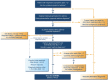
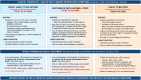
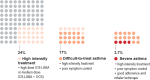
The Global Initiative for Asthma (GINA) was established in 1993 by the World Health Organization and the US National Heart Lung and Blood Institute to improve asthma awareness, prevention and management worldwide. GINA develops and publishes evidence-based, annually updated resources for clinicians. GINA guidance is adopted by national asthma guidelines in many countries, adapted to fit local healthcare systems, practices, and resource availability. GINA is independent of industry, funded by the sale and licensing of its materials. This review summarizes key practical guidance for primary care from the 2022 GINA strategy report. It provides guidance on confirming the diagnosis of asthma using spirometry or peak expiratory flow. GINA recommends that all adults, adolescents and most children with asthma should receive inhaled corticosteroid (ICS)-containing therapy to reduce the risk of severe exacerbations, either taken regularly, or (for adults and adolescents with "mild" asthma) as combination ICS-formoterol taken as needed for symptom relief. For patients with moderate-severe asthma, the preferred regimen is maintenance-and-reliever therapy (MART) with ICS-formoterol. Asthma treatment is not "one size fits all"; GINA recommends individualized assessment, adjustment, and review of treatment. As many patients with difficult-to-treat or severe asthma are not referred early for specialist review, we provide updated guidance for primary care on diagnosis, further investigation, optimization and treatment of severe asthma across secondary and tertiary care. While the GINA strategy has global relevance, we recognize that there are special considerations for its adoption in low- and middle-income countries, particularly the current poor access to inhaled medications.
© 2023. The Author(s).
Conflict of interest statement
J.D. declares no competing interests. The other authors report the following conflicting interests (including financial and non-financial): M.L.L. has received consultancy fees from Clement Clarke International, Boehringer Ingelheim, AstraZeneca, GSK, Orion, TEVA pharmaceuticals, Menarini, NSHI, Chiesi Pharmaceuticals, Novartis, Aspire, Respiri Ltd (Australia), and Smart Respiratory Products Ltd, speaker fees from TEVA, Novartis, Orion, AstraZeneca, NAPP, Chiesi, and NSHI, and honoraria from ADMIT Group - Consorzio Ferrara Ricerche. M.L.L. is Editor Emeritus of
Figures





References
-
- Royal College of Physicians. Why Asthma Still Kills. The National Review of Asthma Deaths (NRAD) Confidential Enquiry Report (RCP, 2014).
-
- Global Asthma Network. The Global Asthma Report 2022. Int. J. Tuberc. Lung Dis. 2022;26:S1–S102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

