TRIM40 is a pathogenic driver of inflammatory bowel disease subverting intestinal barrier integrity
- PMID: 36755029
- PMCID: PMC9908899
- DOI: 10.1038/s41467-023-36424-0
TRIM40 is a pathogenic driver of inflammatory bowel disease subverting intestinal barrier integrity
Abstract
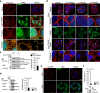
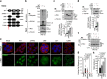
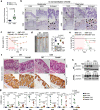
The cortical actin cytoskeleton plays a critical role in maintaining intestinal epithelial integrity, and the loss of this architecture leads to chronic inflammation, as seen in inflammatory bowel disease (IBD). However, the exact mechanisms underlying aberrant actin remodeling in pathological states remain largely unknown. Here, we show that a subset of patients with IBD exhibits substantially higher levels of tripartite motif-containing protein 40 (TRIM40), a gene that is hardly detectable in healthy individuals. TRIM40 is an E3 ligase that directly targets Rho-associated coiled-coil-containing protein kinase 1 (ROCK1), an essential kinase involved in promoting cell-cell junctions, markedly decreasing the phosphorylation of key signaling factors critical for cortical actin formation and stabilization. This causes failure of the epithelial barrier function, thereby promoting a long-lived inflammatory response. A mutant TRIM40 lacking the RING, B-box, or C-terminal domains has impaired ability to accelerate ROCK1 degradation-driven cortical actin disruption. Accordingly, Trim40-deficient male mice are highly resistant to dextran sulfate sodium (DSS)-induced colitis. Our findings highlight that aberrant upregulation of TRIM40, which is epigenetically silenced under healthy conditions, drives IBD by subverting cortical actin formation and exacerbating epithelial barrier dysfunction.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. - PubMed
-
- Wu SK, et al. Cortical F-actin stabilization generates apical-lateral patterns of junctional contractility that integrate cells into epithelia. Nat. Cell Biol. 2014;16:167–178. - PubMed
-
- Chalut KJ, Paluch EK. The actin cortex: a bridge between cell shape and function. Dev. Cell. 2016;38:571–573. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

