Future therapies for cystic fibrosis
- PMID: 36755044
- PMCID: PMC9907205
- DOI: 10.1038/s41467-023-36244-2
Future therapies for cystic fibrosis
Abstract
We are currently witnessing transformative change for people with cystic fibrosis with the introduction of small molecule, mutation-specific drugs capable of restoring function of the defective protein, cystic fibrosis transmembrane conductance regulator (CFTR). However, despite being a single gene disorder, there are multiple cystic fibrosis-causing genetic variants; mutation-specific drugs are not suitable for all genetic variants and also do not correct all the multisystem clinical manifestations of the disease. For many, there will remain a need for improved treatments. Those patients with gene variants responsive to CFTR modulators may have found these therapies to be transformational; research is now focusing on safely reducing the burden of symptom-directed treatment. However, modulators are not available in all parts of the globe, an issue which is further widening existing health inequalities. For patients who are not suitable for- or do not have access to- modulator drugs, alternative approaches are progressing through the trials pipeline. There will be challenges encountered in design and implementation of these trials, for which the established global CF infrastructure is a major advantage. Here, the Cystic Fibrosis National Research Strategy Group of the UK NIHR Respiratory Translational Research Collaboration looks to the future of cystic fibrosis therapies and consider priorities for future research and development.
© 2023. The Author(s).
Conflict of interest statement
Lu. Allen, Lo. Allen, J.T.F., and K.W.S. declare no competing interests. S.B.C. has received grants from the NIHR and undertaking consultancy work for Vertex Pharmaceuticals and Chiesi. She has performed advisory roles for Profile Pharma, Pharmaxis and Vertex Pharmaceuticals. Siobhan is the chair of the UK CF registry steering committee and the European CF society patient registry scientific committee. G.D. has performed clinical trial leadership roles and received speaker honoraria from Vertex Pharmaceuticals, and speaker honoraria from Chiesi Ltd for educational events. She holds current grants from UKRI, NIHR and CF Trust. D.D. has received honoraria and/or grants from, Vertex, Proteostasis, Chiesi, Gilead and Insmed. M.E. is a consultant for Xanadu Bio Sciences. R.G. has received speaker fees from Vertex and Chiesi and and provided consultancy work for Chiesi. C.H. has performed clinical trial leadership roles, and educational and/or advisory activities for 30 Technology, Aradigm, Chiesi, CSL Behring, Gilead, Grifols, GSK, Insmed, Janssen, Meiji, Mylan, Novartis, Pneumagen, Shionogi, Teva, Vertex and Zambon. A.H. has performed clinical trial leadership roles, and educational or advisory activities for the following: Boehringer Ingelheim Pharma GmbH, Celtaxys Pharmaceuticals, Flatley Labs, Galapagos NV, Roche-Genentech, Krystal Biotech, Novabiotics, Pulmocide, Vertex Pharmaceuticals. He holds current grants from EPSRC, UKRI, CF Trust, JP Moulton charity, North West Lung Centre Charity. A.R.S. has received research grants; honoraria for lectures; and support for attending meetings from Vertex Pharmaceuticals (all outside the submitted work). A.R.S. has patents issued (Camara M, Williams P, Barrett D, Halliday N, Knox A, Smyth A, Fogarty A, Barr H, Forrester D. Alkyl quinolones as biomarkers of Pseudomonas aeruginosa infection and uses thereof (
Figures
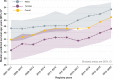
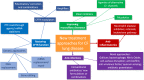
References
-
- Castellani C, Massie J, Sontag M, Southern KW. Newborn screening for cystic fibrosis. Lancet Respir. Med. 2016;4:653–661. - PubMed
-
- Barben J, et al. The expansion and performance of national newborn screening programmes for cystic fibrosis in Europe. J. Cyst. Fibros. 2017;16:207–213. - PubMed
-
- Dijk FN, McKay K, Barzi F, Gaskin KJ, Fitzgerald DA. Improved survival in cystic fibrosis patients diagnosed by newborn screening compared to a historical cohort from the same centre. Arch. Dis. Child. 2011;96:1118–1123. - PubMed
-
- Kerem E, Conway S, Elborn S, Heijerman H, Consensus C. Standards of care for patients with cystic fibrosis: a European consensus. J. Cyst. Fibros. 2005;4:7–26. - PubMed
-
- Castellani C, et al. ECFS best practice guidelines: the 2018 revision. J. Cyst. Fibros. 2018;17:153–178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

