Aberrant phase separation and nucleolar dysfunction in rare genetic diseases
- PMID: 36755093
- PMCID: PMC9931588
- DOI: 10.1038/s41586-022-05682-1
Aberrant phase separation and nucleolar dysfunction in rare genetic diseases
Abstract
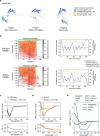
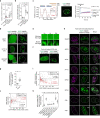
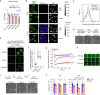
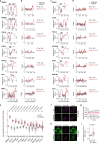
Thousands of genetic variants in protein-coding genes have been linked to disease. However, the functional impact of most variants is unknown as they occur within intrinsically disordered protein regions that have poorly defined functions1-3. Intrinsically disordered regions can mediate phase separation and the formation of biomolecular condensates, such as the nucleolus4,5. This suggests that mutations in disordered proteins may alter condensate properties and function6-8. Here we show that a subset of disease-associated variants in disordered regions alter phase separation, cause mispartitioning into the nucleolus and disrupt nucleolar function. We discover de novo frameshift variants in HMGB1 that cause brachyphalangy, polydactyly and tibial aplasia syndrome, a rare complex malformation syndrome. The frameshifts replace the intrinsically disordered acidic tail of HMGB1 with an arginine-rich basic tail. The mutant tail alters HMGB1 phase separation, enhances its partitioning into the nucleolus and causes nucleolar dysfunction. We built a catalogue of more than 200,000 variants in disordered carboxy-terminal tails and identified more than 600 frameshifts that create arginine-rich basic tails in transcription factors and other proteins. For 12 out of the 13 disease-associated variants tested, the mutation enhanced partitioning into the nucleolus, and several variants altered rRNA biogenesis. These data identify the cause of a rare complex syndrome and suggest that a large number of genetic variants may dysregulate nucleoli and other biomolecular condensates in humans.
© 2023. The Author(s).
Conflict of interest statement
D. Hnisz and X.S. are founders and scientific advisors of Nuage Therapeutics. The other authors declare no competing interests.
Figures










References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

