Polygenic architecture of rare coding variation across 394,783 exomes
- PMID: 36755099
- PMCID: PMC10614218
- DOI: 10.1038/s41586-022-05684-z
Polygenic architecture of rare coding variation across 394,783 exomes
Abstract
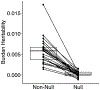
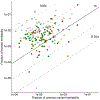
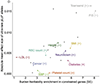
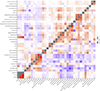
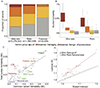
Both common and rare genetic variants influence complex traits and common diseases. Genome-wide association studies have identified thousands of common-variant associations, and more recently, large-scale exome sequencing studies have identified rare-variant associations in hundreds of genes1-3. However, rare-variant genetic architecture is not well characterized, and the relationship between common-variant and rare-variant architecture is unclear4. Here we quantify the heritability explained by the gene-wise burden of rare coding variants across 22 common traits and diseases in 394,783 UK Biobank exomes5. Rare coding variants (allele frequency < 1 × 10-3) explain 1.3% (s.e. = 0.03%) of phenotypic variance on average-much less than common variants-and most burden heritability is explained by ultrarare loss-of-function variants (allele frequency < 1 × 10-5). Common and rare variants implicate the same cell types, with similar enrichments, and they have pleiotropic effects on the same pairs of traits, with similar genetic correlations. They partially colocalize at individual genes and loci, but not to the same extent: burden heritability is strongly concentrated in significant genes, while common-variant heritability is more polygenic, and burden heritability is also more strongly concentrated in constrained genes. Finally, we find that burden heritability for schizophrenia and bipolar disorder6,7 is approximately 2%. Our results indicate that rare coding variants will implicate a tractable number of large-effect genes, that common and rare associations are mechanistically convergent, and that rare coding variants will contribute only modestly to missing heritability and population risk stratification.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Figures



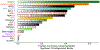




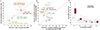
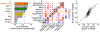

Comment in
-
How rare mutations contribute to complex traits.Nature. 2023 Feb;614(7948):418-419. doi: 10.1038/d41586-023-00272-1. Nature. 2023. PMID: 36755145 No abstract available.
References
Main Text References
Methods References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

