Pleural fluid microbiota as a biomarker for malignancy and prognosis
- PMID: 36755121
- PMCID: PMC9908925
- DOI: 10.1038/s41598-023-29001-4
Pleural fluid microbiota as a biomarker for malignancy and prognosis
Abstract
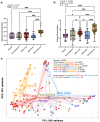
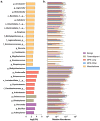
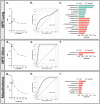
Malignant pleural effusions (MPE) complicate malignancies and portend worse outcomes. MPE is comprised of various components, including immune cells, cancer cells, and cell-free DNA/RNA. There have been investigations into using these components to diagnose and prognosticate MPE. We hypothesize that the microbiome of MPE is unique and may be associated with diagnosis and prognosis. We compared the microbiota of MPE against microbiota of pleural effusions from non-malignant and paramalignant states. We collected a total of 165 pleural fluid samples from 165 subjects; Benign (n = 16), Paramalignant (n = 21), MPE-Lung (n = 57), MPE-Other (n = 22), and Mesothelioma (n = 49). We performed high throughput 16S rRNA gene sequencing on pleural fluid samples and controls. We showed that there are compositional differences among pleural effusions related to non-malignant, paramalignant, and malignant disease. Furthermore, we showed differential enrichment of bacterial taxa within MPE depending on the site of primary malignancy. Pleural fluid of MPE-Lung and Mesothelioma were associated with enrichment with oral and gut bacteria that are commonly thought to be commensals, including Rickettsiella, Ruminococcus, Enterococcus, and Lactobacillales. Mortality in MPE-Lung is associated with enrichment in Methylobacterium, Blattabacterium, and Deinococcus. These observations lay the groundwork for future studies that explore host-microbiome interactions and their influence on carcinogenesis.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

