Sex-divergent effects on the NAD+-dependent deacetylase sirtuin signaling across the olfactory-entorhinal-amygdaloid axis in Alzheimer's and Parkinson's diseases
- PMID: 36755296
- PMCID: PMC9906849
- DOI: 10.1186/s13293-023-00487-x
Sex-divergent effects on the NAD+-dependent deacetylase sirtuin signaling across the olfactory-entorhinal-amygdaloid axis in Alzheimer's and Parkinson's diseases
Abstract
Background: Smell impairment is one of the earliest features in Alzheimer's (AD) and Parkinson's diseases (PD). Due to sex differences exist in terms of smell and olfactory structures as well as in the prevalence and manifestation of both neurological syndromes, we have applied olfactory proteomics to favor the discovery of novel sex-biased physio-pathological mechanisms and potential therapeutic targets associated with olfactory dysfunction.
Methods: SWATH-MS (sequential window acquisition of all theoretical fragment ion spectra mass spectrometry) and bioinformatic workflows were applied in 57 post-mortem olfactory tracts (OT) derived from controls with no known neurological history (n = 6F/11M), AD (n = 4F/13M) and PD (n = 7F/16M) subjects. Complementary molecular analyses by Western-blotting were performed in the olfactory bulb (OB), entorhinal cortex (EC) and amygdala areas.
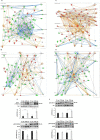
Results: 327 and 151 OT differentially expressed proteins (DEPs) were observed in AD women and AD men, respectively (35 DEPs in common). With respect to PD, 198 DEPs were identified in PD women, whereas 95 DEPs were detected in PD men (20 DEPs in common). This proteome dyshomeostasis induced a disruption in OT protein interaction networks and widespread sex-dependent pathway perturbations in a disease-specific manner, among them Sirtuin (SIRT) signaling. SIRT1, SIRT2, SIRT3 and SIRT5 protein levels unveiled a tangled expression profile across the olfactory-entorhinal-amygdaloid axis, evidencing disease-, sex- and brain structure-dependent changes in olfactory protein acetylation.
Conclusions: Alteration in the OT proteostasis was more severe in AD than in PD. Moreover, protein expression changes were more abundant in women than men independent of the neurological syndrome. Mechanistically, the tangled SIRT profile observed across the olfactory pathway-associated brain regions in AD and PD indicates differential NAD (+)-dependent deacetylase mechanisms between women and men. All these data shed new light on differential olfactory mechanisms across AD and PD, pointing out that the evaluation of the feasibility of emerging sirtuin-based therapies against neurodegenerative diseases should be considered with caution, including further sex dimension analyses in vivo and in clinical studies.
Keywords: Alzheimer; Olfaction; Parkinson; Proteomics; Sexual dimorphism; Sirtuin.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Vassar R, Chao SK, Sitcheran R, Nunez JM, Vosshall LB, Axel R. Topographic organization of sensory projections to the olfactory bulb. Cell. 1994;79(6):981–991. - PubMed
-
- Soussi-Yanicostas N, de Castro F, Julliard AK, Perfettini I, Chedotal A, Petit C. Anosmin-1, defective in the X-linked form of Kallmann syndrome, promotes axonal branch formation from olfactory bulb output neurons. Cell. 2002;109(2):217–228. - PubMed
-
- Doty RL. Olfactory dysfunction in Parkinson disease. Nat Rev Neurol. 2012;8(6):329–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

