Advances in gene therapy hold promise for treating hereditary hearing loss
- PMID: 36755494
- PMCID: PMC10124073
- DOI: 10.1016/j.ymthe.2023.02.001
Advances in gene therapy hold promise for treating hereditary hearing loss
Abstract
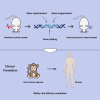
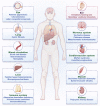
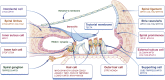
Gene therapy focuses on genetic modification to produce therapeutic effects or treat diseases by repairing or reconstructing genetic material, thus being expected to be the most promising therapeutic strategy for genetic disorders. Due to the growing attention to hearing impairment, an increasing amount of research is attempting to utilize gene therapy for hereditary hearing loss (HHL), an important monogenic disease and the most common type of congenital deafness. Several gene therapy clinical trials for HHL have recently been approved, and, additionally, CRISPR-Cas tools have been attempted for HHL treatment. Therefore, in order to further advance the development of inner ear gene therapy and promote its broad application in other forms of genetic disease, it is imperative to review the progress of gene therapy for HHL. Herein, we address three main gene therapy strategies (gene replacement, gene suppression, and gene editing), summarizing the strategy that is most appropriate for particular monogenic diseases based on different pathogenic mechanisms, and then focusing on their successful applications for HHL in preclinical trials. Finally, we elaborate on the challenges and outlooks of gene therapy for HHL.
Keywords: CRISPR-Cas; clinical trials; gene therapy; genetic diseases; hereditary hearing loss.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
-
- OMIM Gene Map Statistics . 2023. OMIM Morbid Map Scorecard.https://www.omim.org/statistics/geneMap
-
- Milewicz D.M. In: Cardiovascular Medicine. Willerson J.T., Wellens H.J.J., Cohn J.N., Holmes D.R., editors. Springer; 2007. Classification of genetic disorders; pp. 2551–2555. - DOI
-
- Genetic Alliance . 2010. Understanding Genetics: A District of Columbia Guide for Patients and Health Professionals. Appendix G, Single-Gene Disorders.https://www.ncbi.nlm.nih.gov/books/NBK132154/ - PubMed
-
- The Journal of Gene Medicine . 2022. Gene Therapy Clinical Trials Worldwide.https://a873679.fmphost.com/fmi/webd/GTCT
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

