Fasudil alleviates the vascular endothelial dysfunction and several phenotypes of Fabry disease
- PMID: 36755495
- PMCID: PMC10124081
- DOI: 10.1016/j.ymthe.2023.02.003
Fasudil alleviates the vascular endothelial dysfunction and several phenotypes of Fabry disease
Abstract
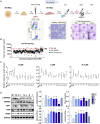
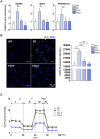
Fabry disease (FD), a lysosomal storage disorder, is caused by defective α-galactosidase (GLA) activity, which results in the accumulation of globotriaosylceramide (Gb3) in endothelial cells and leads to life-threatening complications such as left ventricular hypertrophy (LVH), renal failure, and stroke. Enzyme replacement therapy (ERT) results in Gb3 clearance; however, because of a short half-life in the body and the high immunogenicity of FD patients, ERT has a limited therapeutic effect, particularly in patients with late-onset disease or progressive complications. Because vascular endothelial cells (VECs) derived from FD-induced pluripotent stem cells display increased thrombospondin-1 (TSP1) expression and enhanced SMAD2 signaling, we screened for chemical compounds that could downregulate TSP1 and SMAD2 signaling. Fasudil reduced the levels of p-SMAD2 and TSP1 in FD-VECs and increased the expression of angiogenic factors. Furthermore, fasudil downregulated the endothelial-to-mesenchymal transition (EndMT) and mitochondrial function of FD-VECs. Oral administration of fasudil to FD mice alleviated several FD phenotypes, including LVH, renal fibrosis, anhidrosis, and heat insensitivity. Our findings demonstrate that fasudil is a novel candidate for FD therapy.
Keywords: Fabry disease; drug screening; fasudil; iPSCs; vascular endothelial cells.
Copyright © 2023 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors have declared no competing interests.
Figures





References
-
- Rombach S.M., van den Bogaard B., de Groot E., Groener J.E.M., Poorthuis B.J., Linthorst G.E., van den Born B.J.H., Hollak C.E.M., Aerts J.M.F.G. Vascular aspects of fabry disease in relation to clinical manifestations and elevations in plasma globotriaosylsphingosine. Hypertension. 2012;60:998–1005. doi: 10.1161/HYPERTENSIONAHA.112.195685. - DOI - PubMed
-
- Orteu C.H., Jansen T., Lidove O., Jaussaud R., Hughes D.A., Pintos-Morell G., Ramaswami U., Parini R., Sunder-Plassman G., Beck M., Mehta A.B., FOS Investigators Fabry disease and the skin: data from FOS, the Fabry outcome survey. Br. J. Dermatol. 2007;157:331–337. doi: 10.1111/j.1365-2133.2007.08002.x. - DOI - PubMed
-
- Schiffmann R., Warnock D.G., Banikazemi M., Bultas J., Linthorst G.E., Packman S., Sorensen S.A., Wilcox W.R., Desnick R.J. Fabry disease: progression of nephropathy, and prevalence of cardiac and cerebrovascular events before enzyme replacement therapy. Nephrol. Dial. Transpl. 2009;24:2102–2111. doi: 10.1093/ndt/gfp031. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

