Unlocking the potential of polymeric desalination membranes by understanding molecular-level interactions and transport mechanisms
- PMID: 36755730
- PMCID: PMC9890600
- DOI: 10.1039/d2sc04920a
Unlocking the potential of polymeric desalination membranes by understanding molecular-level interactions and transport mechanisms
Abstract
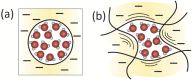
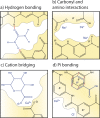
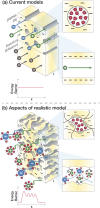
Polyamide reverse osmosis (PA-RO) membranes achieve remarkably high water permeability and salt rejection, making them a key technology for addressing water shortages through processes including seawater desalination and wastewater reuse. However, current state-of-the-art membranes suffer from challenges related to inadequate selectivity, fouling, and a poor ability of existing models to predict performance. In this Perspective, we assert that a molecular understanding of the mechanisms that govern selectivity and transport of PA-RO and other polymer membranes is crucial to both guide future membrane development efforts and improve the predictive capability of transport models. We summarize the current understanding of ion, water, and polymer interactions in PA-RO membranes, drawing insights from nanofiltration and ion exchange membranes. Building on this knowledge, we explore how these interactions impact the transport properties of membranes, highlighting assumptions of transport models that warrant further investigation to improve predictive capabilities and elucidate underlying transport mechanisms. We then underscore recent advances in in situ characterization techniques that allow for direct measurements of previously difficult-to-obtain information on hydrated polymer membrane properties, hydrated ion properties, and ion-water-membrane interactions as well as powerful computational and electrochemical methods that facilitate systematic studies of transport phenomena.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





References
-
- Boretti A. Rosa L. npj Clean Water. 2019;2:15. doi: 10.1038/s41545-019-0039-9. - DOI
-
- Kasperson R. E. and Kasperson J. X., Climate Change, Vulnerability, and Social Justice, in Social Contours of Risk, Routledge, London, England, 2005
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

