Egg Development After In Vitro Insemination in Japanese Quail (Coturnix japonica)
- PMID: 36756046
- PMCID: PMC9884635
- DOI: 10.2141/jpsa.2023001
Egg Development After In Vitro Insemination in Japanese Quail (Coturnix japonica)
Abstract
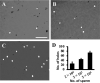
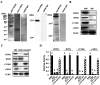
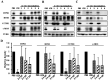
In vitro fertilization has been widely used to produce offspring in several mammalian species. We previously successfully produced Japanese quail chicks using intracytoplasmic sperm injection (ICSI), whereas in vitro insemination was not successful. This may be due to the difficulties associated with mimicking the sperm-egg fusion process and subsequent events in physiological polyspermic fertilization in vitro. In the present study, we observed egg development after in vitro insemination and investigated the inactivation of metaphase-promoting factor (MPF) and cytostatic factor (CSF), which are downstream of the Ca2+ signaling pathway in the egg, due to fertilizing sperm. We found a sperm number-dependent increase in hole formation caused by sperm penetration of the perivitelline membrane, the extracellular coat surrounding the egg. Egg development was observed following in vitro insemination; however, the developmental rate and stages after 24-h culture were inferior to those of ICSI eggs, even when insemination was performed with a high number of sperm (2 × 104). We also noted the downregulation of inositol 1,4,5-trisphosphate receptor-1, ryanodine receptor-3, cyclin B1, and c-MOS, which are important regulatory components of MPF and CSF in the egg, which was dependent on the number of sperm used for insemination. However, the decreases observed in these components did not reach the levels observed in the ICSI eggs. Collectively, the present results suggest that a sperm number higher than 2 × 104 is required for the progression of the Ca2+ signaling pathway, which initiates subsequent egg development in Japanese quail.
Keywords: Blastoderm; Cytostatic factor; In vitro insemination; Japanese quail; Metaphase-promoting factor.
2023 Japan Poultry Science Association.
Conflict of interest statement
Conflicts of Interest: The authors declare no conflict of interest.
Figures
Similar articles
-
Possible involvement of annexin A6 in preferential sperm penetration in the germinal disk region.Reprod Fertil. 2022 Aug 26;3(3):152-161. doi: 10.1530/RAF-21-0115. Print 2022 Jul 1. Reprod Fertil. 2022. PMID: 35972319 Free PMC article.
-
Handling of Gametes for In Vitro Insemination in Birds.Methods Mol Biol. 2017;1650:243-257. doi: 10.1007/978-1-4939-7216-6_16. Methods Mol Biol. 2017. PMID: 28809026
-
The birth of quail chicks after intracytoplasmic sperm injection.Development. 2014 Oct;141(19):3799-806. doi: 10.1242/dev.111765. Development. 2014. PMID: 25249465
-
Application of intracytoplasmic sperm injection (ICSI) for fertilization and development in birds.Gen Comp Endocrinol. 2014 Jan 15;196:100-5. doi: 10.1016/j.ygcen.2013.11.001. Epub 2013 Nov 13. Gen Comp Endocrinol. 2014. PMID: 24239795 Review.
-
Calcium signals for egg activation in mammals.J Pharmacol Sci. 2006;100(5):545-52. doi: 10.1254/jphs.cpj06003x. J Pharmacol Sci. 2006. PMID: 16799264 Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

