The Gradual Release of Alendronate for the Treatment of Critical Bone Defects in Osteoporotic and Control Rats
- PMID: 36756052
- PMCID: PMC9901358
- DOI: 10.2147/IJN.S386784
The Gradual Release of Alendronate for the Treatment of Critical Bone Defects in Osteoporotic and Control Rats
Abstract
Purpose: Osteoporosis is a severe health problem with social and economic impacts on society. The standard treatment consists of the systemic administration of drugs such as bisphosphonates, with alendronate (ALN) being one of the most common. Nevertheless, complications of systemic administration occur with this drug. Therefore, it is necessary to develop new strategies, such as local administration.
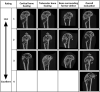
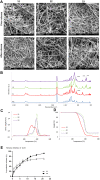
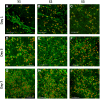
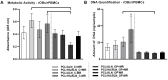
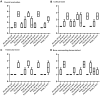
Methods: In this study, emulsion/dispersion scaffolds based on W/O emulsion of PCL and PF68 with ALN, containing hydroxyapatite (HA) nanoparticles as the dispersion phase were prepared using electrospinning. Scaffolds with different release kinetics were tested in vitro on the co-cultures of osteoblasts and osteoclast-like cells, isolated from adult osteoporotic and control rats. Cell viability, proliferation, ALP, TRAP and CA II activity were examined. A scaffold with a gradual release of ALN was tested in vivo in the bone defects of osteoporotic and control rats.
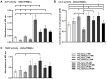
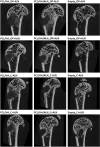
Results: The release kinetics were dependent on the scaffold composition and the used system of the poloxamers. The ALN was released from the scaffolds for more than 22 days. The behavior of cells cultured in vitro on scaffolds with different release kinetics was comparable. The difference was evident between cell co-cultures isolated from osteoporotic and control animals. The PCL/HA scaffold show slow degradation in vivo and residual scaffold limited new bone formation inside the defects. Nevertheless, the released ALN supported bone formation in the areas surrounding the residual scaffold. Interestingly, a positive effect of systemic administration of ALN was not proved.
Conclusion: The prepared scaffolds enabled tunable control release of ALN. The effect of ALN was proved in vitro and in in vivo study supported peri-implant bone formation.
Keywords: alendronate; co-culture; drug delivery system; fibrous scaffold; osteoporosis.
© 2023 Hedvičáková et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

Similar articles
-
Sustained delivery of alendronate by engineered collagen scaffold for the repair of osteoporotic bone defects and resistance to bone loss.J Biomed Mater Res A. 2020 Dec;108(12):2460-2472. doi: 10.1002/jbm.a.36997. Epub 2020 Jun 17. J Biomed Mater Res A. 2020. PMID: 32419333
-
The Effect of Alendronate Loaded Biphasic Calcium Phosphate Scaffolds on Bone Regeneration in a Rat Tibial Defect Model.Int J Mol Sci. 2015 Nov 6;16(11):26738-53. doi: 10.3390/ijms161125982. Int J Mol Sci. 2015. PMID: 26561810 Free PMC article.
-
Enhancing alendronate release from a novel PLGA/hydroxyapatite microspheric system for bone repairing applications.Pharm Res. 2009 Feb;26(2):422-30. doi: 10.1007/s11095-008-9759-0. Epub 2008 Nov 1. Pharm Res. 2009. PMID: 18979188
-
Simultaneous delivery of BMP-2 factor and anti-osteoporotic drugs using hyaluronan-assembled nanocomposite for synergistic regulation on the behaviors of osteoblasts and osteoclasts in vitro.J Biomater Sci Polym Ed. 2015;26(5):290-310. doi: 10.1080/09205063.2014.998588. Epub 2015 Jan 13. J Biomater Sci Polym Ed. 2015. PMID: 25585099
-
How Efficient are Alendronate-Nano/Biomaterial Combinations for Anti-Osteoporosis Therapy? An Evidence-Based Review of the Literature.Int J Nanomedicine. 2022 Dec 6;17:6065-6094. doi: 10.2147/IJN.S388430. eCollection 2022. Int J Nanomedicine. 2022. PMID: 36510618 Free PMC article. Review.
Cited by
-
Electrospun L-Lysine/Amorphous Calcium Phosphate Loaded Core-Sheath Nanofibers for Managing Oral Biofilm Infections and Promoting Periodontal Tissue Repairment.Int J Nanomedicine. 2024 Mar 20;19:2917-2938. doi: 10.2147/IJN.S453702. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38525010 Free PMC article.
-
3D-printed porous zinc scaffold combined with bioactive serum exosomes promotes bone defect repair in rabbit radius.Aging (Albany NY). 2024 May 31;16(11):9625-9648. doi: 10.18632/aging.205891. Epub 2024 May 31. Aging (Albany NY). 2024. PMID: 38829771 Free PMC article.
-
BMP2 peptide-modified polycaprolactone-collagen nanosheets for periodontal tissue regeneration.Front Bioeng Biotechnol. 2025 Mar 5;13:1523735. doi: 10.3389/fbioe.2025.1523735. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40110497 Free PMC article.
-
Electrospun Poly(L-lactide-co-ε-caprolactone) Nanofibers with Hydroxyapatite Nanoparticles Mimic Cellular Interplay in Bone Regeneration.Int J Mol Sci. 2025 Jun 4;26(11):5383. doi: 10.3390/ijms26115383. Int J Mol Sci. 2025. PMID: 40508197 Free PMC article.
References
-
- Henriksen K, Byrjalsen I, Andersen JR, et al. A randomized, double-blind, multicenter, placebo-controlled study to evaluate the efficacy and safety of oral salmon calcitonin in the treatment of osteoporosis in postmenopausal women taking calcium and vitamin D. Bone. 2016;91:122–129. doi:10.1016/j.bone.2016.07.019 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

