Spatiotemporal local and abscopal cell death and immune responses to histotripsy focused ultrasound tumor ablation
- PMID: 36756111
- PMCID: PMC9900174
- DOI: 10.3389/fimmu.2023.1012799
Spatiotemporal local and abscopal cell death and immune responses to histotripsy focused ultrasound tumor ablation
Abstract
Introduction: Histotripsy is a novel focused ultrasound tumor ablation modality with potent immunostimulatory effects.
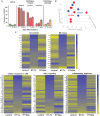
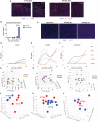
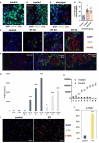
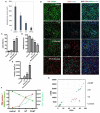
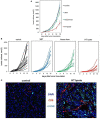
Methods: To measure the spatiotemporal kinetics of local andabscopal responses to histotripsy, C57BL/6 mice bearing bilateral flank B16 melanoma or Hepa1-6 hepatocellular carcinoma tumors were treated with unilateral sham or partial histotripsy. Treated and contralateral untreated (abscopal) tumors were analyzed using multicolor immunofluorescence, digital spatial profiling, RNA sequencing (RNASeq), and flow cytometry.
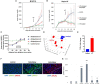
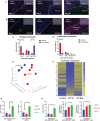
Results: Unilateral histotripsy triggered abscopal tumor growth inhibition. Within the ablation zone, early high mobility group box protein 1 (HMGB1) release and necroptosis were accompanied by immunogenic cell death transcriptional responses in tumor cells and innate immune activation transcriptional responses in infiltrating myeloid and natural killer (NK) cells. Delayed CD8+ T cell intratumoral infiltration was spatiotemporally aligned with cancer cell features of ferroptosis; this effect was enhanced by CTLA-4 blockade and recapitulated in vitro when tumor-draining lymph node CD8+ T cells were co-cultured with tumor cells. Inoculation with cell-free tumor fractions generated by histotripsy but not radiation or freeze/thaw conferred partial protection from tumor challenge.
Discussion: We propose that histotripsy may evoke local necroptotic immunogenic cell death, priming systemic adaptive immune responses and abscopal ferroptotic cancer cell death.
Keywords: ablation; ferroptosis; histotripsy; immunity; necroptosis; tumor; ultrasound.
Copyright © 2023 Pepple, Guy, McGinnis, Felsted, Song, Hubbard, Worlikar, Garavaglia, Dib, Chao, Boyle, Olszewski, Xu, Ganguly and Cho.
Conflict of interest statement
ZX is a stockholder in and consultatant for HistoSonics, Inc. and CC is a consultant on a clinical trial sponsored by HistoSonics, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

