Efficacy and safety results by menopausal status in monarchE: adjuvant abemaciclib combined with endocrine therapy in patients with HR+, HER2-, node-positive, high-risk early breast cancer
- PMID: 36756142
- PMCID: PMC9900651
- DOI: 10.1177/17588359231151840
Efficacy and safety results by menopausal status in monarchE: adjuvant abemaciclib combined with endocrine therapy in patients with HR+, HER2-, node-positive, high-risk early breast cancer
Abstract
Background: Abemaciclib is the first and only cyclin-dependent kinases 4 and 6 inhibitor approved for adjuvant treatment of hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-), node-positive, and high-risk early breast cancer (EBC), with indications varying by geography. Premenopausal patients with HR+, HER2- tumors may have different tumor biology and treatment response compared to postmenopausal patients.
Objectives: We describe the efficacy and safety of abemaciclib plus endocrine therapy (ET) for the large subgroup of premenopausal patients with HR+, HER2- EBC in monarchE.
Design: Randomized patients (1:1) received adjuvant ET with or without abemaciclib for 2 years plus at least 3 additional years of ET as clinically indicated.
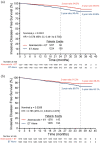
Methods: Patients were stratified by menopausal status (premenopausal versus postmenopausal) at diagnosis. Standard ET (tamoxifen or aromatase inhibitor) with or without gonadotropin-releasing hormone agonist was determined by physician's choice. Invasive disease-free survival (IDFS) and distant relapse-free survival (DRFS) by menopausal status were assessed at data cutoff on 1 April 2021 (median follow-up of 27 months).
Results: Among randomized patients, 2451 (43.5%) were premenopausal and 3181 (56.4%) were postmenopausal. The choice of ET for premenopausal patients varied considerably between countries. Treatment benefit was consistent across menopausal status, with a numerically greater effect size in premenopausal patients. For premenopausal patients, abemaciclib with ET resulted in a 42.2% and 40.3% reduction in the risk of developing IDFS and DRFS events, respectively. Absolute improvement at 3 years was 5.7% for IDFS and 4.4% for DRFS rates. Safety profile for premenopausal patients was consistent with the overall safety population.
Conclusion: Abemaciclib with ET demonstrated clinically meaningful treatment benefit for IDFS and DRFS versus ET alone regardless of menopausal status and first ET, with a numerically greater benefit in the premenopausal compared to the postmenopausal population. Safety data in premenopausal patients are consistent with the overall safety profile of abemaciclib.
Keywords: abemaciclib; early breast cancer; high risk; monarchE; premenopausal.
© The Author(s), 2023.
Conflict of interest statement
SPS reports fees for from Pfizer for corporate-sponsored research; fees for speaker’s bureau, honoraria, consultancy from Roche, Novartis, Pfizer, and AstraZeneca; fees for consultancy from Eli Lilly and Company and Gilead; and fees for speaker’s bureau and consultancy from MSD, outside the submitted work. PN reports institutional fees for consultancy from Pfizer, Novartis, Eli Lilly and Company, Roche, and Astrazeneca; institutional fees for advisory board participation from Pfizer, Novartis, Eli Lilly and Company, Roche, and Sanofi; institutional fees for lecturing and attendance at scientific exchange meetings, and research funding from Kom op Tegan Kanker, outside the submitted work. JH receives fees for advisory board participation from Eli Lilly and Company, Novartis, Roche, Pfizer, Hexal, AstraZeneca, MSD, Celgene, and Abbvie; fees for corporate-sponsored research from Celgene, Novartis, Hexal, and Eli Lilly and Company; and fees for travel expenses from Roche, Pfizer, Novartis, Celgene, and Daiichi Sankyo, outside the submitted work; IC has nothing to disclose, outside the submitted work; MPG reports institutional fees for consultancy from Eli Lilly and Company, Biovica, Novartis, Sermonix, Context Pharm, Pfizer, AstraZeneca, Eagle Pharmaceuticals; personal fees from Genomic Health; institutional grant from Pfizer, Eli Lilly and Company, Sermonix; institutional honoraria for advisory board participation from ARC Therapeutics, Blueprint Medicines, Sanofi Genzyme, Biotheranostics; personal fees for CME presentation from Research to Practice, Clinical Education Alliance, Medscape; personal fees for session moderating from Curio Science; personal fees for serving as a panelist from Total Health Conferencing, outside the submitted work; CS reports grants for corporate-sponsored research from Eli Lilly and Company, outside the submitted work. CSH reports personal fees and grants for advisory board participation and speaker’s bureau from Eli Lilly and Company, Novartis, and Daiichi Sankyo; grants, personal fees, and non-financial support for advisory board participation, and speaker’s bureau from Pfizer, Roche and AstraZeneca; grants from EirGenix and OBI Pharma, grants and personal fees for speaker’s bureaus from MSD; outside the submitted work. HJL reports fees for corporate-sponsored research and travel support from Eli Lilly and Company, outside the submitted work. JB reports institution fees for advisory board participation from Eli Lilly and Company, Roche, and Pfizer, outside the submitted work. ET reports personal fees for lectures from Eli Lilly and Company, AstraZeneca and Daiichi Sankyo, outside the submitted work. JRC reports corporate-sponsored research fees from Eli Lilly and Company. ROS reports corporate-sponsored research fees from Eli Lilly and Company. RW, AS, SCN, TF are employees and stock shareholders of Eli Lilly and Company. SRDJ reports personal fees for consulting, advisory role, speaker’s bureau, and research funding from AstraZeneca, Novartis, Pfizer; personal fees for consulting, advisory role, research funding from Eli Lilly and Company, Puma Biotechnology; personal fees for speaker’s bureau from Eisai; and personal fees for speaker’s bureau, research funding from Roche/Genentech, outside the submitted work. NH reports personal fees for lectures and consulting from AstraZeneca, Eli Lilly and Company, Novartis, and Pfizer, outside the submitted work.
Figures


References
-
- Harbeck N, Rastogi P, Martin M, et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol 2021; 32: 1571–1581. - PubMed
-
- Partridge AH, Hughes ME, Warner ET, et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol 2016; 34: 3308–3314. - PubMed
-
- Zhong W, Tan L, Jiang WG, et al. Effect of younger age on survival outcomes in T1N0M0 breast cancer: a propensity score matching analysis. J Surg Oncol 2019; 119: 1039–1046. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

