Visible light mediated organocatalytic dehydrogenative aza-coupling of 1,3-diones using aryldiazonium salts
- PMID: 36756411
- PMCID: PMC9853514
- DOI: 10.1039/d2ra07807d
Visible light mediated organocatalytic dehydrogenative aza-coupling of 1,3-diones using aryldiazonium salts
Abstract
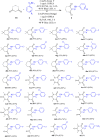
An efficient protocol for diazenylation of 1,3-diones under photoredox conditions is presented herein. C-N bond forming Csp3 -H functionalization of cyclic and alkyl diones by unstable aryl diazenyl radicals is achieved through reaction with aryldiazonium tetrafluoroborates by organocatalysts under visible light irradiation. The reaction has wide substrate scope, gives excellent yields, and is also efficient in water as a green solvent. This method provides an easy access to aryldiazenyl derivatives that are useful key starting materials for the synthesis of aza heterocycles as well as potential pharmacophores.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






Similar articles
-
Merging Visible Light Photoredox and Gold Catalysis.Acc Chem Res. 2016 Oct 18;49(10):2261-2272. doi: 10.1021/acs.accounts.6b00351. Epub 2016 Sep 9. Acc Chem Res. 2016. PMID: 27610939
-
Visible-Light-Induced C(sp2)-C(sp3) Cross-Dehydrogenative-Coupling Reaction of N-Heterocycles with N-Alkyl-N-methylanilines under Mild Conditions.J Org Chem. 2021 Sep 3;86(17):11723-11735. doi: 10.1021/acs.joc.1c01207. Epub 2021 Aug 9. J Org Chem. 2021. PMID: 34369160
-
Direct arylation of N-heteroarenes with aryldiazonium salts by photoredox catalysis in water.Chemistry. 2014 Mar 3;20(10):2960-5. doi: 10.1002/chem.201304120. Epub 2014 Feb 5. Chemistry. 2014. PMID: 24500947
-
Organocatalytic Synthesis of Highly Functionalized Heterocycles by Enantioselective aza-Morita-Baylis-Hillman-Type Domino Reactions.Chem Pharm Bull (Tokyo). 2020;68(4):299-315. doi: 10.1248/cpb.c19-00900. Chem Pharm Bull (Tokyo). 2020. PMID: 32238648 Review.
-
Recent advances in organocatalytic asymmetric aza-Michael reactions of amines and amides.Beilstein J Org Chem. 2021 Oct 18;17:2585-2610. doi: 10.3762/bjoc.17.173. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 34760026 Free PMC article. Review.
Cited by
-
TiO2-Catalyzed Direct Diazenylation of Active Methylene Compounds with Diazonium Salts.J Org Chem. 2025 Jan 10;90(1):300-308. doi: 10.1021/acs.joc.4c02266. Epub 2024 Dec 17. J Org Chem. 2025. PMID: 39690472 Free PMC article.
References
-
- Beharry A. A. Woolley G. A. Chem. Soc. Rev. 2011;40:4422–4437. - PubMed
- Szymański W. Beierle J. M. Kistemaker H. A. V. Velema W. A. Feringa B. L. Chem. Rev. 2013;113:6114–6178. - PubMed
- Dong M. Babalhavaeji A. Samanta S. Beharry A. A. Woolley G. A. Acc. Chem. Res. 2015;48:2662–2670. - PubMed
- Hartwig J. F. Nature. 2008;455:314–322. - PMC - PubMed
- Yudin A. K. and Hartwig J. F., Catalysed Carbon-Heteroatom Bond Formation, Wiley-VCH, Weinheim, 2010
- Yasukawa N. Kuwata M. Imai T. Monguchi Y. Sajiki H. Sawama Y. Green Chem. 2018;20:4409–4413.
- Shi J. Yuan T. Wang R. Zheng M. Wang X. Green Chem. 2021;23:3945–3949.
-
- Yu Y. Nakano M. Ikeda T. Nature. 2003;425:145. - PubMed
- Sondhi S. M. Dinodia M. Kumar A. Bioorg. Med. Chem. 2006;14:4657–4663. - PubMed
- Fehrentz T. Schönberger M. Trauner D. Angew. Chem., Int. Ed. 2011;50:12156–12182. - PubMed
- Beharry A. A. Woolley G. A. Chem. Soc. Rev. 2011;40:4422–4437. - PubMed
- Ho B. K. Ngaini Z. Matthew Neilsen P. Hwang S. S. Entigu Linton R. Kong E. L. Lee B. K. J. Chem. 2017;2017:1–7.
- Lee S. H. Moroz E. Castagner B. Leroux J. C. J. Am. Chem. Soc. 2014;136:12868–12871. - PubMed
- Sofan M. A. El-Mekabaty A. Hasel A. M. Said S. B. J. Heterocycl. Chem. 2021;58:1645–1655.
- Kumar G. S. Neckers D. C. Chem. Rev. 1989;89:1915–1925.
-
- Roldo M. Barbu E. Brown J. F. Laight D. W. Smart J. D. Tsibouklis J. Expert Opin. Drug Delivery. 2007;4:547–560. - PubMed
- Cassano R. Trombino S. Cilea A. Ferrarelli T. Muzzalupo R. Picci N. Chem. Pharm. Bull. 2010;58:103–105. - PubMed
- Jain A. Gupta Y. Jain S. K. Crit. Rev. Ther. Drug Carrier Syst. 2006;23:349–400. - PubMed
-
- Benkhaya S. M’rabet S. El Harfi A. Heliyon. 2020;6:e03271. - PMC - PubMed
- Koshti S. M. Sonar J. P. Sonawane A. E. Pawar Y. A. Nagle P. S. Mahulikar P. P. More D. H. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 2008;47:329–331.
- Zhao R. Tan C. Xie Y. Gao C. Liu H. Jiang Y. Tetrahedron Lett. 2011;52:3805–3809.
- Grirrane A. Corma A. García H. Science. 2008;322:1661–1664. - PubMed
- Qiao R. Z. Zhang Y. Hui X. P. Xu P. F. Zhang Z. Y. Wang X. Y. Green Y. L. Chem. 2001;3:186–188.
- Zhang C. Jiao N. Angew. Chem. 2010;122:6310–6313.
-
- Deng B. Li C. Yuan J. Rao C. B. Zhao Y. Zhang R. Dong D. Tetrahedron. 2019;75:2273–2279.
- Raju G. B. Mahesh M. Manjunath G. Ramana P. V. Chem. Sci. Trans. 2016;5:125–136.
- dos Santos Filho J. M. de Queiroz e Silva D. M. A. Macedo T. S. Teixeira H. M. P. Moreira D. R. M. Challal S. Wolfender J. L. Queiroz E. F. Soares M. B. P. Bioorg. Med. Chem. 2016;24:5693–5701. - PubMed
- Pranali D. Kale J. Chem. Pharm. Res. 2013;5:130–134.
LinkOut - more resources
Full Text Sources

