DNA repair in cardiomyocytes is critical for maintaining cardiac function in mice
- PMID: 36756698
- PMCID: PMC10014058
- DOI: 10.1111/acel.13768
DNA repair in cardiomyocytes is critical for maintaining cardiac function in mice
Abstract
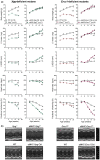
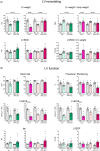
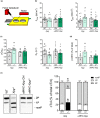
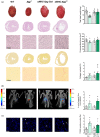
Heart failure has reached epidemic proportions in a progressively ageing population. The molecular mechanisms underlying heart failure remain elusive, but evidence indicates that DNA damage is enhanced in failing hearts. Here, we tested the hypothesis that endogenous DNA repair in cardiomyocytes is critical for maintaining normal cardiac function, so that perturbed repair of spontaneous DNA damage drives early onset of heart failure. To increase the burden of spontaneous DNA damage, we knocked out the DNA repair endonucleases xeroderma pigmentosum complementation group G (XPG) and excision repair cross-complementation group 1 (ERCC1), either systemically or cardiomyocyte-restricted, and studied the effects on cardiac function and structure. Loss of DNA repair permitted normal heart development but subsequently caused progressive deterioration of cardiac function, resulting in overt congestive heart failure and premature death within 6 months. Cardiac biopsies revealed increased oxidative stress associated with increased fibrosis and apoptosis. Moreover, gene set enrichment analysis showed enrichment of pathways associated with impaired DNA repair and apoptosis, and identified TP53 as one of the top active upstream transcription regulators. In support of the observed cardiac phenotype in mutant mice, several genetic variants in the ERCC1 and XPG gene in human GWAS data were found to be associated with cardiac remodelling and dysfunction. In conclusion, unrepaired spontaneous DNA damage in differentiated cardiomyocytes drives early onset of cardiac failure. These observations implicate DNA damage as a potential novel therapeutic target and highlight systemic and cardiomyocyte-restricted DNA repair-deficient mouse mutants as bona fide models of heart failure.
Keywords: DNA damage; DNA repair; apoptosis; cardiac function; congestive heart failure.
© 2023 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
XPG in the Nucleotide Excision Repair and Beyond: a study on the different functional aspects of XPG and its associated diseases.Mol Biol Rep. 2022 Aug;49(8):7995-8006. doi: 10.1007/s11033-022-07324-1. Epub 2022 May 20. Mol Biol Rep. 2022. PMID: 35596054 Review.
-
Loss of DNA repair mechanisms in cardiac myocytes induce dilated cardiomyopathy.Aging Cell. 2023 Apr;22(4):e13782. doi: 10.1111/acel.13782. Epub 2023 Feb 3. Aging Cell. 2023. PMID: 36734200 Free PMC article.
-
Postnatal growth failure, short life span, and early onset of cellular senescence and subsequent immortalization in mice lacking the xeroderma pigmentosum group G gene.Mol Cell Biol. 1999 Mar;19(3):2366-72. doi: 10.1128/MCB.19.3.2366. Mol Cell Biol. 1999. PMID: 10022922 Free PMC article.
-
Deficiency in the nuclease activity of xeroderma pigmentosum G in mice leads to hypersensitivity to UV irradiation.Mol Cell Biol. 2004 Mar;24(6):2237-42. doi: 10.1128/MCB.24.6.2237-2242.2004. Mol Cell Biol. 2004. PMID: 14993263 Free PMC article.
-
XPG: a multitasking genome caretaker.Cell Mol Life Sci. 2022 Mar 1;79(3):166. doi: 10.1007/s00018-022-04194-5. Cell Mol Life Sci. 2022. PMID: 35230528 Free PMC article. Review.
Cited by
-
Replication-Independent ICL Repair: From Chemotherapy to Cell Homeostasis.J Mol Biol. 2024 Jul 1;436(13):168618. doi: 10.1016/j.jmb.2024.168618. Epub 2024 May 18. J Mol Biol. 2024. PMID: 38763228 Free PMC article. Review.
-
Obesity accelerates cardiovascular ageing.Eur Heart J. 2025 Jun 16;46(23):2161-2185. doi: 10.1093/eurheartj/ehaf216. Eur Heart J. 2025. PMID: 40197620 Free PMC article. Review.
-
Impact of diffused versus vasculature targeted DNA damage on the heart of mice depleted of telomeric factor Ft1.Aging Cell. 2023 Dec;22(12):e14022. doi: 10.1111/acel.14022. Epub 2023 Nov 13. Aging Cell. 2023. PMID: 37960940 Free PMC article.
-
Hybrid Molecular and Functional Micro-CT Imaging Reveals Increased Myocardial Apoptosis Preceding Cardiac Failure in Progeroid Ercc1 Mice.Mol Imaging Biol. 2024 Aug;26(4):628-637. doi: 10.1007/s11307-024-01902-4. Epub 2024 Mar 18. Mol Imaging Biol. 2024. PMID: 38498063 Free PMC article.
-
Ercc1 DNA repair deficiency results in vascular aging characterized by VSMC phenotype switching, ECM remodeling, and an increased stress response.Aging Cell. 2024 May;23(5):e14126. doi: 10.1111/acel.14126. Epub 2024 Mar 7. Aging Cell. 2024. PMID: 38451018 Free PMC article.
References
-
- Agah, R. , Frenkel, P. A. , French, B. A. , Michael, L. H. , Overbeek, P. A. , & Schneider, M. D. (1997). Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac‐restricted, site‐specific rearrangement in adult ventricular muscle in vivo. The Journal of Clinical Investigation, 100(1), 169–179. 10.1172/JCI119509 - DOI - PMC - PubMed
-
- Ataei Ataabadi, E. , Golshiri, K. , van der Linden, J. , de Boer, M. , Duncker, D. J. , Jüttner, A. , de Vries, R. , van Veghel, R. , van der Pluijm, I. , Dutheil, S. , Chalgeri, S. , Zhang, L. , Lin, A. , Davis, R. E. , Snyder, G. L. , Danser, A. H. J. , & Roks, A. J. M. (2021). Vascular ageing features caused by selective DNA damage in smooth muscle cell. Oxidative Medicine and Cellular Longevity, 2021, 2308317. 10.1155/2021/2308317 - DOI - PMC - PubMed
-
- Barnhoorn, S. , Uittenboogaard, L. M. , Jaarsma, D. , Vermeij, W. P. , Tresini, M. , Weymaere, M. , Menoni, H. , Brandt, R. M. , de Waard, M. C. , Botter, S. M. , Sarker, A. H. , Jaspers, N. G. , van der Horst, G. , Cooper, P. K. , Hoeijmakers, J. H. , & van der Pluijm, I. (2014). Cell‐autonomous progeroid changes in conditional mouse models for repair endonuclease XPG deficiency. PLoS Genetics, 10(10), e1004686. 10.1371/journal.pgen.1004686 - DOI - PMC - PubMed
-
- Bartunek, J. , Vanderheyden, M. , Knaapen, M. W. , Tack, W. , Kockx, M. M. , & Goethals, M. (2002). Deoxyribonucleic acid damage/repair proteins are elevated in the failing human myocardium due to idiopathic dilated cardiomyopathy. Journal of the American College of Cardiology, 40(6), 1097–1103; discussion 1104‐1095. 10.1016/s0735-1097(02)02122-8 - DOI - PubMed
-
- Bautista‐Nino, P. K. , Portilla‐Fernandez, E. , Rubio‐Beltran, E. , van der Linden, J. J. , de Vries, R. , van Veghel, R. , de Boer, M. , Durik, M. , Ridwan, Y. , Brandt, R. , Essers, J. , Menzies, R. I. , Thomas, R. , de Bruin, A. , Duncker, D. J. , van HMM, B. , Ghanbari, M. , JHJ, H. , Sedlacek, R. , … Roks, A. J. M. (2020). Local endothelial DNA repair deficiency causes aging‐resembling endothelial‐specific dysfunction. Clinical Science, 134(7), 727–746. 10.1042/CS20190124 - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

